Physical and chemical properties of water
Basic characteristics of water[edit | edit source]
Water is a two-element compound of oxygen and hydrogen with the general formula H2O. In addition to being the most abundant compound on Earth, it is the most biologically important polar solvent. It covers 70.7% of the earth's surface. It is necessary for all living organisms. Water in living organisms occupies more than half (approx. 60–99%) of their volume, depending on the type of organism. Participates in thermoregulation, substance transfer pH, removes waste products of metabolism and acts as a reaction environment. As an essential component of the human body, it is found in a number of tissues including: blood, sweat, urine, gastric and intestinal juices.
State[edit | edit source]
There are three states of water in nature:
- Liquid - the largest amount (oceans, seas, rivers...)
- Gaseous (water vapor) - in the atmosphere
- Solid (ice, snow, hail...)
In nature, pressure and temperature can change the state of water - a condition of the water cycle.
Physical properties[edit | edit source]
Water is a colorless, clear liquid, tasteless and odorless.
- The melting point is 0 °C, the boiling point is 100 °C, which are the basic points of the Celsius temperature scale.
- Density increases from 0 °C to 3.98 °C, then decreases with increasing temperature. As the temperature decreases, the density decreases again, inversely proportional to the increasing volume. For this reason, ice floats on water (it has a lower density than water), unlike all ordinary substances, where the rule applies that when the temperature increases, the volume increases and when the temperature decreases, it decreases in all temperature intervals.
- The greatest density of water (1g/cm3) is at 3.98 °C.
→This phenomenon is called a water anomaly. It is important for aquatic animals. A layer of ice forms on the surface of the water, which prevents further freezing.
- Ice has a larger volume than liquid water. When melting, its volume decreases, when it solidifies, it increases.
- Explanation: The crystal structure of ice contains channels where meltwater flows when the crystal structure is disrupted. The disorder of liquid water molecules in the channels causes a smaller volume than it would be in the case of an ordered crystal lattice.
- The relative volume increase is 9%.
- increase in volume has a negative effect especially when freezing in crevices → shattering of rocks, cracking of masonry, etc.
- Explanation: The crystal structure of ice contains channels where meltwater flows when the crystal structure is disrupted. The disorder of liquid water molecules in the channels causes a smaller volume than it would be in the case of an ordered crystal lattice.
- Viscosity decreases with increasing temperature. From value viscosity is derived from the speed of water filtration through sand or sedimentation in wastewater treatment plants.
(At 0 °C: 1.78 mPa.s, at 100 °C: 0.28 mPa.s)
- Surface tension decreases with increasing temperature. The surface tension of water is the second highest of common substances. Important in capillary phenomena. It is reduced by chemical detergents.
(At 0 °C: 75.6 mN/m, at 100 °C: 58.9 mN/m)
- Water has a very low thermal conductivity, which prevents water from freezing to greater depths.
- Electrical conductivity depends on the content of ions in the water. Pure water is very poorly electrically conductive. With the addition of ions, the electrical conductivity of water increases significantly.
- The specific heat capacity of water is relatively high. The phenomenon is used in central heating.
- Water also has a specific high heat of vaporization, which is of great importance for removing heat from the surface of the body through perspiration.
Chemical properties[edit | edit source]
- Water is one of the most stable compounds. It decomposes into hydrogen and oxygen only at high temperatures.
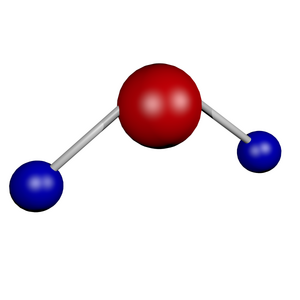
- The shape is a tetrahedron with an angle of 105°. The shape is given by the 2 lone electron pairs on the oxygen. Oxygen carries a partial (partial) negative charge and hydrogens a partial positive charge. It is responsible for the uneven distribution of electron density between hydrogens and oxygen electronegativity.
- The presence of hydrogens allows the formation of hydrogen bridges. They affect chemical and physical properties, especially polarity, melting point, boiling point and state.
- H2O molecule contains 2 covalent sigma (σ) bonds.
- The whole molecule is polar due to the polarity of the bonds between O and H and the dipole moment.
→Water is an important polar solvent – it dissolves substances:
– polar, with an ionic bond – splits in water to form hydrated ions (electrolytic dissociation)
– gaseous (NH3, CO2, O2, hydrogen halides,...)
– non-polar (sugars, alcohols)
- In nature, there is no pure water regularly, but with a certain amount of dissolved substances. We divide it according to the content of dissolved substances:
– Salty x sweet
– Mineral
– Hard x soft
– Distilled – chemically pure
- Hydrates are crystalline substances containing bound water molecules. For example: Blue, white or green rock.
- Distilled water has a pH equal to 7. Depending on the dissolved substances, the pH changes.
- Water in reactions can be:
- Reaction reactant: H2O + SO2→H2SO3
- Reaction product (neutralization): HCl + NaOH→NaCl+H2O
- Reaction medium (hydrolysis): CN– + H2O↔HCN+ OH-
Water hardness[edit | edit source]
hardness is a frequent obstacle in the normal use of water. It is caused by some soluble salts calcium and magnesium. We distinguish between two types of water hardness. Temporary and permanent hardness.
- Temporary hardness of water is mostly caused by bicarbonates and can be removed by boiling.
Ca(HCO3)2 −> CaCO3 + H2O + CO2
CaCO3 is called scale.
- Permanent hardness of water is mainly caused by sulfates and chlorides. It can be removed by adding a softener (Na2CO3–soda)
CaSO4 +Na2CO3 −> Na2SO4 +CaCO3
Links[edit | edit source]
Related articles[edit | edit source]
Sources[edit | edit source]
- Taken from with the author's permission https://uloz.to/!CM6zAi6z/biofot-doc.
- LEDVINA, Miroslav, et al. Biochemistry for medical students. 2nd edition. Prague: Karolinum, 2009. 548 pp. pp. 85-90.ISBN 978-80-246-1414-4.
Internet resources[edit | edit source]
- Nabla.cz, [cit. 24-11-2019], http://www.nabla.cz/obsah/fyzika/molekulova-fyzika-a-termika/tepelna-kapacita-merna-tepelna-kapacita.php
- Webnode.cz, [cit. 24-11-2019], https://voda-organismy.webnode.cz/fyzykalni-a-chemicke-vlastnosti/
- Wikipedia, [cit. 24-11-2019],https://cs.wikipedia.org/wiki/Led
- Wikipedia.cz, [cit. 24-11-2019], https://cs.wikipedia.org/wiki/Vodn%C3%AD_pára
- Wikipedia.cz, [cit. 24-11-2019], https://cs.wikipedia.org/wiki/Voda
- Mining and Geological Faculty VŠB, [cit. 24-11-2019], http://hgf10.vsb.cz/546/Ekologicke%20aspekty/voda/fyzykalni/hydrog_vaz.htm
- Department of Physics Přf OU, [cit. 24-11-2019], http://artemis.osu.cz/MMi/Skerko/DIPLCELA/Diplhtm2/222.htm
- Encyclopedia of Physics, Jaroslav Reichl, Martin Všetička, [cit. 24-11-2019], http://fyzika.jreichl.com/main.article/view/650-zmena-objemu-teles-pri-tani-a-tuhnuti-zavislost-teploty-tani-na-tlaku
- Všichivsem.cz, [cit. 24-11-2019], http://www.vsichnivsem.cz/strednipredmet-149-18hodina