Oxygen toxicity
Oxygen is commonly used to treat pulmonary and extrapulmonary diseases in the form of oxygen therapy or hyperbaric oxygen therapy (pressure > 1 atmosphere). The supply of oxygen to the tissues depends on adequate ventilation, gas exchange and distribution through the blood circulation. When breathing air at normal atmospheric pressure, most of the oxygen is bound to hemoglobin and only a small amount is freely dissolved in the plasma. In hyperoxia, the hemoglobin is completely saturated with oxygen and the amount of dissolved oxygen rises - with normobaric exposure to 100% oxygen, free dissolved oxygen covers about 1/3 of tissue needs. When applying oxygen in a hyperbaric chamber with a pressure of 3 atmospheres, only the oxygen dissolved in the plasma will cover all the needs of the tissues. This effect is used, for example, in carbon monoxide poisoning, when the ability of hemoglobin to bind oxygen is impaired.[1]
Oxygen toxicity is caused by reactive oxygen species, which can damage tissues by inducing necrosis or apoptosis, if the amount of reactive oxygen species exceeds the antioxidant ability of the organism. Premature infants requiring oxygen therapy, patients requiring hyperbaric oxygen therapy, and divers are most at risk of oxygen toxicity. Short exposure to high partial pressures at higher than atmospheric pressure leads to central nervous system damage (diving, hyperbaric oxygen therapy). While long-term exposure to elevated oxygen levels at normal atmospheric pressure primarily damages the lungs and eyes (e.g. premature newborns requiring oxygen therapy).[1]
Consequences of oxygen therapy and hyperoxia[edit | edit source]
- oxygen induces vasodilation of the pulmonary vessels and, conversely, vasoconstriction in the systemic circulation;
- high oxygen concentrations indirectly improve the inflammatory response by alleviating tissue hypoxia;
- during oxygen therapy of acute decompensation of COPD, hypercapnic respiratory failure may occur (→ severe respiratory acidosis → coma), a similar condition may occur in children with severe neuromuscular diseases with chronic respiratory failure;
- inhaling oxygen with an increased partial pressure has a harmful effect - cell damage is most noticeable in the CNS, lungs and eyes.[1]
Mechanism of oxygen toxicity in hyperoxia[edit | edit source]
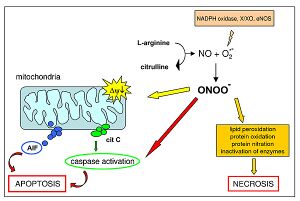
- hyperoxia leads to the formation of oxygen radicals - have one or more unpaired electrons, are very unstable
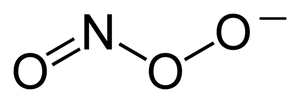
- the most important reactive forms of oxygen include hydroxyl ion (HO–) and peroxynitrite (ONOO–)
- peroxynitrite interacts with lipids, DNA and proteins directly through oxidative reactions and indirectly through mechanisms mediated by radicals → it can cause oxidative cell damage up to cell necrosis or apoptosis;
- there are many antioxidant mechanisms in the organism, but their capacity is exceeded at high concentrations of free oxygen and oxidative tissue damage occurs.[1]
Clinical implications of oxygen toxicity[edit | edit source]
- Lung damage
- the respiratory system is exposed to the highest concentrations of oxygen in the entire organism, therefore the lungs are the first organ where oxygen toxicity manifests itself;
- long-term exposure to elevated oxygen levels at normal atmospheric pressure damages the lungs;
- the rate of damage is directly proportional to the partial pressure of inhaled oxygen;
- at an oxygen fraction < 50% oxygen toxicity usually does not occur;
- 1st manifestation is tracheobronchial irritation/inflammation - substernal or pleural pain (in a matter of hours when inhaling 100% oxygen), cough, dyspnoea, reduced cilia activity → reduced vital capacity, formation of atelectasis, diffuse alveolar damage (image ARDS) → chronic pulmonary fibrosis and emphysema with tachypnea and progressive hypoxemia;
- artificial pulmonary ventilation and possibly primary disease requiring oxygen therapy, then possible pharmacotherapy – for example, dexamethasone in animal models worsens lung damage and reduces the activity of antioxidant enzymes in the lungs; oxygen toxicity is further increased by adrenaline, estrogen, amphetamines and thyroid hormones.
- Damage to the CNS
- damage to the CNS occurs only at very high partial pressure (diving, hyperbaric chamber)
- the speed of progression is directly proportional to the partial pressure (with 4-5 atmospheres, manifestation after only 10 minutes)
- 1st manifestation is visual disturbances (tunnel vision), tinnitus, nausea, twitching of facial muscles, dizziness, confusion (image is variable) → tonic-clonic convulsions (disappears when the partial pressure of oxygen is reduced), impaired consciousness.
- Visual damage
- long-term exposure to elevated oxygen levels at normal atmospheric pressure
- in premature newborns, it is involved in the development of retinopathy of prematurity on the basis of abnormal ingrowth of blood vessels through the immature retina
- divers and patients treated in a hyperbaric chamber may develop hyperoxemic myopia (reversible).
- Damage to other organs
- destruction of erythrocytes, damage to the myocardium, endocrine glands (adrenal glands, gonads and thyroid glands) and kidneys.[1]
References
Related Articles[edit | edit source]
- Oxygen therapy • Hyperbaric oxygen therapy • Oxygen • Oxygen radicals • Oxygen parameters • Antioxidant protection of the human body