Methods of Staining Bacteria, Micromycetes and Parasites
The basic methods of microbiology include the preparation of a native or fixed specimen.
Native Sample[edit | edit source]
Unstained native samples are not widely used in bacteriology. Native samples are used to diagnose parasitic and fungal infections. In bacteriology, the native sample is used, for example, to observe moving Treponema pallidum using dark field microscopy.
The procedure for preparing a native sample is simple. Drop a drop of saline onto the slide and add the sample through the loop. Then cover the whole with a coverslip (without bubbles) and observe with a microscope.
Fixed Sample[edit | edit source]
The sample is fixed before staining. Add saline to the slide and add the sample to this drop with a loop. We then let it dry. Finally, the prepared sample is fixed with a torch flame or, for example, methanol. The purpose of fixation is to kill the bacteria and attach them firmly to the slide so that they do not wash during the subsequent staining.
Staining Techniques[edit | edit source]
Staining of slides is performed after fixation of the sample. The coloration of individual structures of microorganisms facilitates their identification.
Immersion objective microscopy with 1000x magnification is used for bacteria with a size of several µm. Drizzle the slide on a slide with a drop of immersion oil, the coverslip is not used. Then immerse the lens in immersion oil. At the end of the observation, it is necessary to clean the lens (with gasoline-moistened pulp).
Types:
- Gram stain
- Ziehl-Neelsen Acid Fast Stain
- Burri's India Ink Method
- Staining metachromatic granules using Albert's Stain
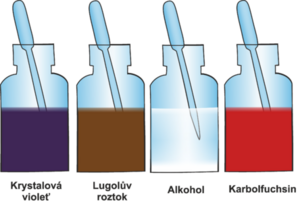
Gram Staining[edit | edit source]
Gram staining divides bacteria into gram-positive and gram-negative. This coloring is quick and easy.
The principle is the absorption of the crystal violet complex with Lugol's solution (iodine solution in potassium iodide) into bacterial cells. The division of bacteria into two groups is based on the different elution of this complex with an organic solvent (acetone) from the bacterial wall. So a better name would be Gram's discoloration. Different discoloration occurs due to the different structure of the bacterial wall. The bacterial wall contains peptidoglycan murein.
The wall of gram-negative bacteria is thin, it contains a large amount of lipids. The thin wall allows better penetration of the organic solvent and easy washing of the crystal violet complex from bacteria. Therefore, the wall must be colored pink with carbolfuchsin.
The wall of gram-positive bacteria is thicker and stratified. The thick wall retains the crystal violet in the bond with iodine and there is no leaching. Gram-positive bacteria turn dark blue to bluish-purple thanks to crystal violet.
Gram staining procedure:
- Drip crystal violet on the prepared fixed specimen and let it work.
- After 30 seconds, rinse with water..
- Then we add Lugol's solution, which contains iodine (iodine forms a complex with crystal violet).
- After 30 seconds, rinse with water.
- Then apply a solvent (ethanol or acetone).
- After 30 seconds, rinse with water– gram-positive bacteria remain stained due to the thick wall, gram-negative bacteria discolour.
- Finally, we add carbolfuchsin or safranin for contrast and coloration.
- After 30 seconds, rinse with water - gram-positive bacteria remain the same color, gram-negative bacteria turn pink.
Ziehl-Neelsen Acid Fast Stain[edit | edit source]
Acid-fast staining is intended for acid-resistant bacteria of the genus Mycobacterium and Nocardia. According to Gram, these microorganisms do not stain due to a cell wall that contains lipids with mycolic acids. These lipids do not absorb dyes poorly and must therefore be dyed hot (dyes so absorbed cannot be washed off even by decolorization with solvents containing strong acids (acid resistance) or bases).
Acid-resistant bacteria are colored pink to red with carbolfuchsin. The contrasting background is green or blue.
Ziehl-Neelsen staining procedure:
- Carbolfuchsin is applied to the fixed preparation and heated with a torch (hot dyeing).
- Subsequently, we decolorize the specimen with acid alcohol while tilting the slide.
- Rinse the specimen in running water.
- Then stain the slide with malachite green (or methylene blue) for 30 seconds.
- Rinse the specimen in running water.
- Leave to dry
Giemsa Stain[edit | edit source]
The principle is the absorption of Giemsa dye, which is based on eosin and methylene blue. The sample can be a thick blood drop or a fixed vaginal swab. Searched organisms include protozoa, bacteria, rickettsiae, chlamydia, mycoplasma viral inclusions. Bacteria turn blue, cases light blue, mucus pink.
The fixed sample is immersed in concentrated or diluted Giemsa solution for 2 minutes, 30 minutes or overnight as required. Subsequently, the specimen is rinsed with water, dried and can be observed with an immersion lens.
Albert's Stain[edit | edit source]
Albert staining is used to represent metachromatic granules. The fixed slide is overlaid with Albert's solution, then rinsed with water and dried. The preparation is then poured over Lugol's solution and rinsed again and dried. Bacteria are stained green, metachromatic granules are blue. It is mainly used with Corynebacterium spp.
Writz – Conklin Stain[edit | edit source]
Writz-Conclin staining is used to depict bacterial spores, such as the spores of Clostridium tetanii. The fixed sample is overlaid with a solution of malachite green and, after rinsing, is stained with dilute carbolfuchsin. The bacterial cell is pink, the spores are green.
Burri's India Ink Method[edit | edit source]
Burri staining is used to detect bacterial envelopes. A drop of the sample is mixed with ink on a slide. Then a thin coat is made, which is allowed to dry. The preparation is stained with crystal violet. The bacteria turn purple (thanks to crystal violet). The case does not stain, which creates a halo effect around the bacteria, which borders the cell from the black environment, which is colored with ink.
Staining Micromycetes[edit | edit source]
Candida albicans and the genus Aspergillus are stained according to Gram. Gentian violet is a specific dye for Zygomycetes. Cryptococcus is a yeast with a polysaccharide capsule, so it stains according to Burri.
Staining in Parasitology[edit | edit source]
Giemsa-Romanowski staining and Gomori trichrome staining are used. A dry lens with 4x magnification is used for parasites with a size of tens to hundreds of µm. Ziehl-Neelsen staining is used for intestinal coccidia(Toxoplasma gondii).
Fluorescent Staining[edit | edit source]
Non-specific fluorescence is used in mycology, where a fluorescent dye stains chitin in the cell wall of fungi. Fluorescent staining is also used in the diagnosis of tuberculosis.
Links[edit | edit source]
Related Articles[edit | edit source]
Bibliography[edit | edit source]
- HORÁČEK, Jiří. Základy lékařské mikrobiologie : (obecná a speciální epidemiologie infekčních nemocí). 1. edition. Karolinum, 2000. vol. 1. ISBN 80-246-0006-4.
- GOERING, Richard V – DOCKRELL, Hazel M. Základy lékařské mikrobiologie : (obecná a speciální epidemiologie infekčních nemocí). 5. edition. Triton, 2016. pp. 568. ISBN 978-80-7387-928-0.