Intestinal permeability luclozomanitol test
Intestinal Permeability Tests[edit | edit source]
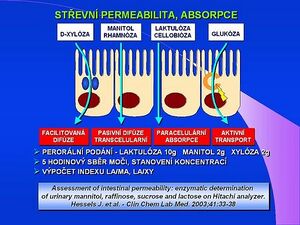
Intestinal permeability tests are suitable in the diagnosis of diseases small intestine, malabsorptive syndrome, especially celiac disease. A whole range of markers can be used to assess intestinal permeability (monosaccharides, disaccharides, 51Cr-EDTA, polyethyleneglycol – PEG) or their combination. The lactulose/mannitol test (LA/MA test) is the most widely used in our country, combinations with cellobiose or sucrose are described. Lactulose is a marker of paracellular absorption, and in celiac disease, for example, there is a loosening of cellular bonds, an expansion of the tight zone and an increase in the permeability of lactulose. Mannitol is a marker of active transport through the cell (enterocyte) and in celiac disease the absorption of mannitol is reduced by the reduction of the active surface in atrophy of the intestinal mucosa. The LA/MA intestinal permeability test can be used to monitor postoperative chemotherapy.
Lactulose/mannitol test[edit | edit source]
It can also be combined with the administration of D-xylose, this version of the test was developed at the ÚKBD in Hradec Králové. A solution of 10 g of lactulose, 2 g of mannitol, 2 g of D-xylose and 11 g of glucose in 100 ml of distilled water is administered orally, the hyperosmolarity of the solution of 1500 mosm/l increases the sensitivity of the test. The patient must fast (12 hours, usually overnight) and collect urine for 5 hours after drinking the test solution. The analysis of individual sugars is carried out by the technique of gas chromatography, the relative amount of individual sugars in proportion to the administered amount and the final permeability indices LA/MA and LA/XY are calculated. The average value of the LA/MA index in the control group is 0.016 ± 0.008, the LA/XY index is 0.013 ± 0.009. Some other studies recommend wider combinations of the three substrates, e.g. with the administration of sucralose, which is stable during large intestine passage, other studies recommend a combined permeability and lactase test - LDI/SAT index.
Links[edit | edit source]
References[edit | edit source]
- MELICHAR, B. , et al. Intestinal permeability and vitamin A absorption in patients with chemotherapy-induced diarrhea. Am J Clin Oncol. 2008, vol. 31, no. 6, p. 580-4, ISSN 0277-3732 (Print), 1537-453X (Electronic). PMID: 19060591.
- VILELA, EG. , et al. Intestinal permeability and antigliadin antibody test for monitoring adult patients with celiac disease. Dig Dis Sci. 2007, vol. 52, no. 5, p. 1304-9, ISSN 0163-2116 (Print), 1573-2568 (Electronic). PMID: 17356917.
- KOETSE, HA. , et al. Combined LDI/SAT test to evaluate intestinal lactose digestion and mucosa permeability. Eur J Clin Invest. 2006, vol. 36, no. 10, p. 730-6, ISSN 0014-2972 (Print), 1365-2362 (Electronic). PMID: 16968469.
- ANDERSON, AD. , et al. A simple method for the analysis of urinary sucralose for use in tests of intestinal permeability. Ann Clin Biochem. 2005, vol. 42, p. 224-6, ISSN 0004-5632 (Print), 1758-1001 (Electronic). PMID: 15949159.
- DUERKSEN, DR. , et al. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci. 2005, vol. 50, no. 4, p. 785-90, ISSN 0163-2116 (Print), 1573-2568 (Electronic). PMID: 15844719.
- ZUCKERMAN, MJ. , et al. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci. 2004, vol. 49, no. 4, p. 621-6, ISSN 0163-2116 (Print), 1573-2568 (Electronic). PMID: 15185867.
- SECONDULFO, M. , et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. 2004, vol. 36, no. 1, p. 35-45, ISSN 1590-8658 (Print), 1878-3562 (Electronic). PMID: 14971814.
- DI LEO, V. , et al. Lactulose/mannitol test has high efficacy for excluding organic causes of chronic diarrhea. Am J Gastroenterol. 2003, vol. 98, no. 10, p. 2245-52, ISSN 0002-9270 (Print), 1572-0241 (Electronic). PMID: 14572575.
- HESSELS, J. , et al. Assessment of intestinal permeability: enzymatic determination of urinary mannitol, raffinose, sucrose and lactose on Hitachi analyser. Clin Chem Lab Med. 2003, vol. 41, no. 1, p. 33-8, ISSN 1434-6621 (Print). PMID: 12636047.
- INUTSUKA, S. , et al. Assessment of the intestinal permeability following postoperative chemotherapy for human malignant disease. Eur Surg Res. 2003, vol. 35, no. 1, p. 22-5, ISSN 0014-312X (Print), 1421-9921 (Electronic). PMID: 12566783.
- MELICHAR, B. , et al. Intestinal permeability in patients with chemotherapy-induced stomatitis. J Cancer Res Clin Oncol. 2001, vol. 127, no. 5, p. 314-8, ISSN 0171-5216 (Print), 1432-1335 (Electronic). PMID: 11355146.
- KOHOUT, P. Small bowel permeability in diagnosis of celiac disease and monitoring of compliance of a gluten-free diet (gut permeability in celiac disease). Acta Medica (Hradec Kralove). 2001, vol. 44, no. 3, p. 101-4, ISSN 1211-4286. PMID: 11811077.
- VOGELSANG, H. , et al. In vivo and in vitro permeability in celiac disease. Aliment Pharmacol Ther. 2001, vol. 15, no. 9, p. 1417-25, ISSN 0269-2813 (Print), 1365-2036 (Electronic). PMID: 11552914.
- JOHNSTON, SD. , et al. Intestinal permeability tests in celiac disease. Clin Lab. 2001, vol. 47, no. 3-4, p. 143-50, ISSN 1433-6510. PMID: 11294577.
Source[edit | edit source]
- taken from KOCNA, Petr. GastroLab : MiniEncyclopedia of laboratory methods in gastroenterology [online]. ©2002. The last revision 2011-01-08, [cit. 2011-03-04]. <http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm>.