Induction of fetal lung maturity
From WikiLectures
Induction of fetal lung maturity[edit | edit source]
- antenatal administration of corticosteroids in women with preterm labor reduces the risk of neonatal death, the risk of neonatal respiratory distress syndrome, and the risk of fetal/newborn intraventricular hemorrhage;
- betamethasone 12 mg i.m. every 24 hours for a total of two doses or dexamethasone 6 mg i.m. every 12 hours for a total of 4 doses;
- between the 24th and 35th week of pregnancy, if we expect a premature birth in the next 7 days;
- the onset of the full effect of corticosteroids can be expected only after approx. 24 hours after the completion of the entire course, however, the administration of even one dose is important;
- if the effect of corticosteroids has worn off, or if more than 14 days have passed since the previous course, it is possible to administer another course of corticosteroids before the 32nd week of pregnancy and in anticipation of early delivery;
- compensated diabetes mellitus or chorioamnionitis is not a contraindication.[1]
Surfactant[edit | edit source]
- SURFace ACTive AgeNT
- significance: reduces surface tension and promotes stability of alveoli during exhalation → prevents atelectasis, reduces work of breathing, prevents fluid transudation from capillaries into alveolar spaces;
- composition: phospholipids, neutral lipids and surfactant proteins A, B and C (SP-A, SP-B, SP-C) – hydrophilic and hydrophobic components;
- phospholipids: mainly phosphatidylcholine (PC or lecithin, L), phosphatidylglycerol (PG) and to a lesser extent other phospholipids;
- neutral lipids: cholesterol, triacylglycerols (TAG), free fatty acids (FFA);
- sphingomyelin (its concentration does not change during pregnancy), glycolipids and carbohydrates form a very small fraction of surfactant;
- SP-A is a large glycoprotein;
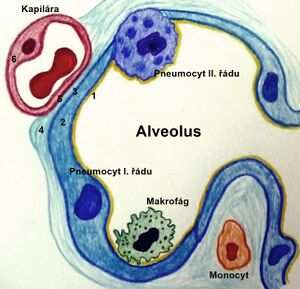
- biosynthesis of surfactant (phosphatidylcholine) takes place in the endoplasmic reticulum of pneumocytes II. type, then it is transported to the lamellar bodies and from there secreted into the alveoli;
- surfactant recycling takes place in alveoli – surfactant degradation in alveoli and small airways → surfactant components are absorbed by pneumocytes and recycled;
- regulation of surfactant production: stretch receptors, stimulation by the entry of gases into the lungs (distension of the alveoli); β-adrenergic receptors;
- pneumocytes I. and II. type can be detected histologically around the 22nd week of pregnancy;[2] pneumocytes II. type makes up about 2% of the alveolar surface;
- in pneumocytes II. type, lamellar bodies gradually appear - intracellularly stored surfactant (around the 24th week of pregnancy);
- factors affecting lung maturation:
- glucocorticoids – endogenous cortisol is an important physiological stimulus of fetal lung maturation;
- beta-adrenergic drugs;
- thyroid hormones – thyroxine increases surfactant production and lung maturation; thyroxine (T4) does not cross the placenta, unlike TRH and triiodothyronine (T3);
- prolactin – unclear significance in the regulation of surfactant production; epidermal growth factor; fibroblast pneumocyte factor;
- insulin – slows down the maturation of pneumocytes II. type and surfactant formation, inhibits SP-A gene expression;
- testosterone – slows down lung maturation and surfactant production.[3]
Assessment of lung maturity[edit | edit source]
The lungs of the fetus secrete a fluid that is excreted into the amniotic fluid. Part of this fluid is surfactant, the composition of which changes during pregnancy.
- ratio of lecithin to sphingomyelin (both are fractions of surfactant; while the concentration of lecithin in surfactant rises during pregnancy, the concentration of sphingomyelin remains the same)
- ratio of lecithin to sphingomyelin (both are fractions of surfactant; while the concentration of lecithin in surfactant rises during pregnancy, the concentration of sphingomyelin remains the same)
- L:S ratio > 2 is usually associated with lung maturation (95% predicts absence of RDS but may be associated with RDS in mothers with diabetes and hemolytic disease of the fetus);
- an L:S ratio < 2 predicts RDS with only 54% accuracy; the lower the L:S, the higher the risk of developing RDS;
- phosphatidylglycerol (PG) concentration
- children with PG rarely have RDS
- the combination of a low L:S ratio with the absence of PG in the amniotic fluid up to 3 days before delivery is a better predictor of the length of ventilatory support than gestational age or birth weight;
- the number of lamellar bodies in the amniotic fluid
- SP-A level in serum in the first 24 hours of life or in amniotic fluid.[3]
These examinations are not currently used in clinical practice.
Sources[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
References[edit | edit source]
- ↑ Česká gynekologická a porodnická společnost. Spontánní předčasný porod : Doporučený postup [online]. ©2017. [cit. 2020-10-23]. <https://www.gynultrazvuk.cz/data/clanky/6/dokumenty/p-2017-spontanni-predcasny-porod.pdf>.
- ↑ JANOTA, Jan – STRAŇÁK, Zbyněk. Neonatologie. 1. edition. Mladá fronta, 2013. pp. 90. ISBN 978-80-204-2994-0.
- ↑ a b RENNIE, JM, et al. Textbook of Neonatology. 5. edition. Churchill Livingstone Elsevier, 2012. pp. 459-464. ISBN 978-0-7020-3479-4.