Immunochemical test for blood in feces
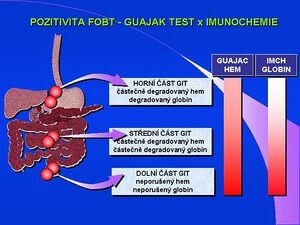
Immunochemical detection of blood in the stool (iFOBT) is intended, unlike the screening guaiac test (Haemoccult, gFOBT), to rule out bleeding into the GIT. The test is based on immunochemical detection of hemoglobin in reactions with monoclonal antibody against human hemoglobin. Sensitivity and positive identification is also significantly affected by the different degradation of both components of hemoglobin with respect to the proximodistal gradient in the digestive system. Globin is degraded much more rapidly, and a positive immunochemical test almost eliminates the detection of bleeding in the upper part of the alimentary canal. Hemagglutination, latex immunoprecipitation, radial immunodiffusion and immunoaffinity chromatography tests are based on the immunochemical principle. Detection of the protein (human hemoglobin) monoclonal antibody excludes the possibility of being affected by another source of hemoglobin (food), the interference of chemical substances is eliminated, and a special diet is not necessary. The sensitivity of immunochemical tests is significantly higher; depending on the technique and < 0.1 mg hemoglobin/g stool. Immunochemical tests include, for example, the latex Hemolex test, Heme-Select based on the principle of reverse passive hemagglutination, ImmoCare immunoaffinity chromatography, Dialab FOB test, Hexagon OBTI, Actim test and others.
Test Execution[edit | edit source]
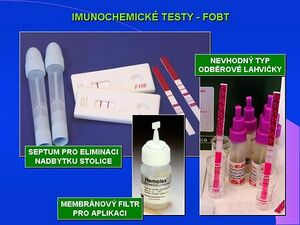
Immunochemical tests vary significantly according to the type of technique used. Recently, the most widespread variant is immunoaffinity chromatography. The patient collects 1 stool sample in a collection container with a stabilizing solution. However, Stool sampling involves a significant risk of preanalytical error. Laboratory processing consists of applying a drop of extract to the test and reading 1 or 2 colored strips that detect the presence of only the antibody with a colored marker (negative test, 1 colored strip) or the formation of an antigen-antibody complex (positive test, 2 colored strips). The evaluation is again only qualitative.
Studies have been testing several immunochemical analyzers for quantitative determination of hemoglobin in stool (qi-FOBT) in recent years, most of which are of Japanese manufacture. ROC curves demonstrate a specificity for advanced adenomass of 95.3% at a sensitivity of 100 ng Hb/mL.
Links[edit | edit source]
Source[edit | edit source]
- KOCNA, Peter. GastroLab : MiniEncyclopedia of laboratory methods in gastroenterology [online]. ©2002. The last revision 2011-01-08, [cit. 2011-03-04]. <http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm>.
References[edit | edit source]
- FRASER, CG. , et al. Evaluation of a card collection-based faecal immunochemical test in screening for colorectal cancer using a two-tier reflex approach. Gut. 2007, vol. 56, no. 10, p. 1415-8, ISSN 0017-5749 (Print), 1468-3288 (Electronic). PMID: 17309886.
- CIATTO, S. , et al. Association of FOBT-assessed faecal Hb content with colonic lesions detected in the Florence screening program. Br J Cancer. 2007, vol. 96, no. 2, p. 218-21, ISSN 0007-0920 (Print), 1532-1827 (Electronic). PMID: 17211476.
- LEVI, Z. , et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007, vol. 146, no. 4, p. 244-55, ISSN 0003-4819 (Print), 1539-3704 (Electronic). PMID: 17310048.
- FRASER, CG. , et al. Immunochemical testing of individuals positive for guaiac faecal occult blood test in a screening program for colorectal cancer: an observational study.. Lancet Oncol. 2006, vol. 7, no. 2, p. 127-31, ISSN 1470-2045 (Print), 1474-5488 (Electronic). PMID: 16455476.
- ROZEN, P. , et al. Evaluation of a desk top instrument for the automated development and immunochemical quantification of fecal occult blood. Med Sci Monit. 2006, vol. 12, no. 6, p. MT27-32, ISSN 1234-1010 (Print), 1643-3750 (Electronic). PMID: 16733493.
- LI, S. , et al. New immunochemical fecal occult blood test with two-consecutive stool sample testing is a cost-effective approach for colon cancer screening: results of a prospective multicenter study in Chinese patients. Int J Cancer. 2006, vol. 118, no. 12, p. 3078-83, ISSN 0020-7136 (Print), 1097-0215 (Electronic). PMID: 16425283.
- GREENWALD, B. From guaiac to immune fecal occult blood tests: the emergence of technology in colorectal cancer screening.. Gastroenterol Nurs. 2005, vol. 28, no. 2, p. 90-6, ISSN 1042-895X (Print), 1538-9766 (Electronic). PMID: 15832108.
- MORIKAWA, T. , et al. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005, vol. 129, no. 2, p. 422-8, ISSN 0016-5085 (Print), 1528-0012 (Electronic). PMID: 16083699.
- BAMPTON, PA. , et al. Interval faecal occult blood testing in a colonoscopy-based screening program detects additional pathology. Gut. 2005, vol. 54, no. 6, p. 803-6, ISSN 0017-5749 (Print), 1468-3288 (Electronic). PMID: 15888788.
- VOGEL, T. , et al. Comparison of different stool tests for the detection of cancer of the colon. Dtsch Med Wochenschr. 2005, vol. 130, no. 14, p. 872-7, ISSN 0012-0472 (Print), 1439-4413 (Electronic). PMID: 15800820.
- BARDOT, L. , et al. Faecal occult blood testing: comparison of a latex agglutination test (Hemolex) and an immunoturbidimetric test (QuikRead FOB). Ann Biol Clin (Paris). 2004, vol. 62, no. 3, p. 339-43, ISSN 0003-3898. PMID: 15217769.
- BERCHI, C. , et al. VCost-effectiveness analysis of two strategies for mass screening for colorectal cancer in France. Health Econ. 2004, vol. 13, no. 3, p. 227-38, ISSN 1057-9230 (Print), 1099-1050 (Electronic). PMID: 14981648.
- NAKAJIMA, M. , et al. Prevention of advanced colorectal cancer by screening using the immunochemical faecal occult blood test: a case-control study. Br J Cancer. 2003, vol. 89, no. 1, p. 23-8, ISSN 0007-0920 (Print), 1532-1827 (Electronic). PMID: 12838295.
- WONG, BC. , et al. A sensitive guaiac faecal occult blood test is less useful than an immunochemical test for colorectal cancer screening in a Chinese population. Aliment Pharmacol Ther. 2003, vol. 18, no. 9, p. 941-6, ISSN 0269-2813 (Print), 1365-2036 (Electronic). PMID: 14616158.
- WONG, WM. , et al. Evaluation of an automated immunochemical fecal occult blood test for colorectal neoplasia detection in a Chinese population. Cancer. 2003, vol. 97, no. 10, p. 2420-4, ISSN 0008-543X (Print), 1097-0142 (Electronic). PMID: 12733140.
- DVORAK, M. , et al. Occult fecal blood loss--comparison of immunochemical and biochemical tests. Cas Lek Cesk. 2002, vol. 141, no. 7, p. 217-9, ISSN 0008-7335. PMID: 12053757.
- HAREWOOD, GC. , et al. Detection of occult upper gastrointestinal tract bleeding: performance differences in fecal occult blood tests. Mayo Clin Proc. 2002, vol. 77, no. 1, p. 23-8, ISSN 0025-6196 (Print), 1942-5546 (Electronic). PMID: 11794453.
- NAKAMA, H. , et al. Evaluation of the optimum cut-off point in immunochemical occult blood testing in screening for colorectal cancer. Eur J Cancer. 2001, vol. 37, no. 3, p. 398-401, ISSN 0014-2964. PMID: 11239763.
- SAITO, H. , et al. A case-control study evaluating occult blood screening for colorectal cancer with hemoccult test and an immunochemical hemagglutination test. Oncol Rep. 2000, vol. 7, no. 4, p. 815-9, ISSN 1021-335X (Print), 1791-2431 (Electronic). PMID: 10854550.
- CASTIGLIONE, G. , et al. Screening for colorectal cancer by faecal occult blood test: comparison of immunochemical tests. J Med Screen. 2000, vol. 7, no. 1, p. 35-7, ISSN 0969-1413 (Print), 1475-5793 (Electronic). PMID: 10807145.