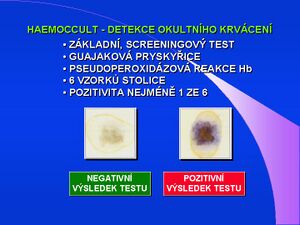
Haemoccult
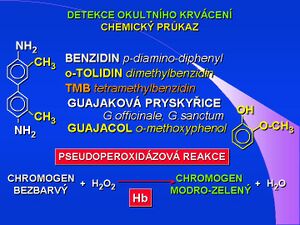
Haemoccult or guaiac occult bleeding test is used to screen for colorectal cancer. Standard occult bleeding tests - TOKS, Haemoccult test, are based on the chemical principle, the pseudoperoxidase reaction of hemoglobin. The substrate was originally benzidine, as a carcinogen, it was modified to non-carcinogenic dimethylbenzidine-o-tolidine (eg German KryptoHaem), or tetramethylbenzidine (TMB). Today, tests with guaiac resin - from Guajacum Officinale / sanctum - containing guaiacol (o-methoxyphenol), a benzidine-type substrate, are used. In the leucoform it is colorless, oxidized turns blue-green. Oxidation takes place with hydrogen peroxide, the catalyst is hemoglobin with pseudoperoxidase activity. Due to the chemical principle of the oxidation reaction, the test result may be affected by the presence of other oxidizing substances (vitamin C), the presence of hemoglobin from food (meat, blood), false-positive result may be caused by the presence of plant peroxidases (some root vegetables). The guidelines of the Ministry of Health of the Czech Republic for the search and early diagnosis of colorectal tumors - screening - clearly specify Haemoccult as a suitable, recommended and verified test. Controlled TOKS screening significantly reduces the incidence of colorectal cancer.
Performing the test[edit | edit source]
A kit is supplied for screening, which contains 3 tests with two windows and plastic or wooden stools for stool collection. The patient always takes two different samples from three consecutive stools, which he spreads on the marked test sites, closes the test windows and sends the tests to the laboratory. Laboratory processing consists in applying the detection reagent to the opposite side of the windows and evaluating any color change. The evaluation is qualitative, each test where a specific blue-green coloration is evaluated is positive. The Haemoccult test sensitivity / cut-off value is set at about 5 mg Hb / g stool. In the study performed at ÚKBLD 1. LF UK and VFN in> 95,000 asymptomatic persons, the Haemoccult test posed a positivity of 2.8%, the false positivity tested in comparison with immunochemical tests was zero.
Home-care test[edit | edit source]
The EZ Detect test, a 2-minute chromogenic test designed for home use, is also based on the chemical principle of blood detection in the stool. The patient performs the test and evaluates it. The test is made of biodegradable material, the substrate is tetramethylbenzidine (TMB).
Resousces[edit | edit source]
Literature[edit | edit source]
- HEWITSON, P. , et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008, y. 103, no. 6, p. 1541-9, ISSN 0002-9270 (Print), 1572-0241 (Electronic). PMID: 18479499.
- CRUZ-CORREA, M. , et al. Performance characteristics and comparison of two fecal occult blood tests in patients undergoing colonoscopy. Dig Dis Sci. 2007, y. 52, no. 4, p. 1009-13, ISSN 0163-2116 (Print), 1573-2568 (Electronic). PMID: 17380401.
- BERCHI, C. , et al. Cost-effectiveness analysis of two strategies for mass screening for colorectal cancer in France. Health Econ. 2004, y. 13, no. 3, p. 227-38, ISSN 1057-9230 (Print), 1099-1050 (Electronic). PMID: 14981648.
- RASMUSSEN, M. , et al. Diagnostic yield in a biennial Hemoccult-II screening program compared to a once-only screening with flexible sigmoidoscopy and Hemoccult-II. Scand J Gastroenterol. 2003, y. 38, no. 1, p. 114-8, ISSN 0036-5521 (Print), 1502-7708 (Electronic). PMID: 12608473.
- JORGENSEN, OD. , et al. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002, y. 50, no. 1, p. 29-32, ISSN 0017-5749 (Print), 1468-3288 (Electronic). PMID: 11772963.
- JOUVE, JL. , et al. Estimation of screening test (Hemoccult) sensitivity in colorectal cancer mass screening. Br J Cancer. 2001, y. 84, no. 11, p. 1477-81, ISSN 0007-0920 (Print), 1532-1827 (Electronic). PMID: 11384097.
- KRISTINSSON, J. , et al. Screening of first degree relatives of patients operated for colorectal cancer: evaluation of fecal calprotectin vs. hemoccult II. Digestion. 2001, y. 64, no. 2, p. 104-10, ISSN 0012-2823 (Print), 1421-9867 (Electronic). PMID: 11684824.
- TOWLER, BP. , et al. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev. 2000, y. -, no. 2, p. CD001216, ISSN 1469-493X (Electronic). PMID: 10796760.
- MANDEL, JS. , et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999, y. 91, no. 5, p. 434-7, ISSN 0027-8874 (Print), 1460-2105 (Electronic). PMID: 10070942.
Related artical[edit | edit source]
- with permission of the author taken from KOCNA, Petr. GastroLab : MiniEncyklopedie laboratorních metod v gastroenterologii [online]. ©2002. The last revision 2011-01-08, [cit. 2011-03-04]. <http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm>.