Inherited metabolic disorders
(Redirected from Hereditary metabolic disorders)
Inherited metabolic disorders (IMDs)[1]
[2]
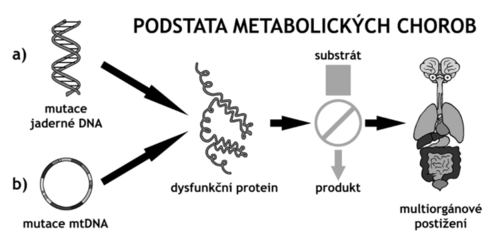
form a diverse group of 700-800 diseases that are caused by enzyme deficiency, transport protein dysfunction or a disorder of another protein associated with a metabolic pathway. They are characterized by autosomal recessive, gonosomal recessive and dominant, but also mitochondrial inheritance [1]. Insufficient production of the enzyme or the required protein occurs as a result of mutations in nuclear or mitochondrial DNA. Conservative estimates the culmulative incidences vof all inherited metabolic disorders are reported to be around 1:500[1][† 1] (heterozygote frequency 1:15[3]); it is very likely that IMDs are currently underdiagnosed[3]. Data from neinatal screening programs can provide an illustration of their true incidence, eg in the US the cumulative incidence for 50 screened DMPs ranges from 1:1000 to 1:2000[† 2].
Although the group of inherited metabolic disorders is highly heterogeneous, some commonalities can ve found. It follows from their very nature that biochemical and enzymatic abnormalities will be detectable in patients[2]. Furthermore, because most metabolic pathways are common to many cells in the body, multiorgan involvement is common (eg. CNS, muscle, kidney and liver involvement in mitochondrial diseases[1]). Clinical manifestations of DMP are very often non-specific (failure, loss of appetite, growth disorder, porucha psychomotor development disorder, disorders of consciousness), only very rarely do specific signs of a high probability for some IMDs (eg. sweaty foot odor in patients with isovaleric aciduria or typical facial dysmorphia in patients with mucopolysaccharidoses or generalized peroxisomal diseases). They affect patients of any age from the prenatal period to old age.
Causes
The most common cause of inherited metabolic disorders are nuclear DNA mutations in germ cells (and thus in somatic cells) with typical monogenic Mendelian inheritance — usually autosomal recessive, gonosomal recessive and dominant. A less common cause of IMD is mitochondrial DNA mutations, which are transmitted by the maternal type of inheritance. Phenotypic manifestations in two individuals with the same genotype may differ due to other factors such as environmental influences (diet, lifestyle in dieta, životní style in small molecule diseases) or such as epigenic changes, epistasis (interactions with allelic variants in other genes), inactivation X-chromosome (lyonization). Mutations can be of the type of point mutations (missense, nonsense, synonymous mutations), deletions and insertions (with or without frameshift), and the degree of impairment of a given protein often cannot be directly determined from the type of mutation and its location.
The mutation can result in an altered amount of translated protein (usually decreased or rarely increased) or its properties (by a change in the isolated function of one domain, or by a global change in all functions, eg. in misfolding). Mutations can also lead to changes in the function of non-protein gene products such as miRNAs or siRNAs, which regulate the expression of a number of target genes.
The affected protein is usually an enzyme of some metabolic pathway, which then binds to its product, which may be missing, and does not drain the substrate, which may accumulate, or metabolize to by-product. From this, the involvement of different organs derives to different degrees.
Affected metabolic pathways
Inherited metabolic disorders typically include metabolic disorders:
- disorders of organelle metabolisml
- mitochondrial disease
- peroxisomal disease
- lysosomal disease
- glycosylation disorders (bound to the endoplasmic reticulum)
- metabolic disorders not primarily bound to organelles
- other IMD
Manifestations
The fundamental difference in the nature of the metabolites that cause the clinical manifestations of the disease allows inherited metabolic disorders to be divided into two groups:
- Disease of small molecules
- Diseases of complex moleculesl
Diseases of small molecules
Diseases of small molecules are caused by the accumulation of small toxic molecules (ammonia, organic acids) or lack of desirable metabolites (ketone bodies, glucose), which are caused by catabolism of food-borne substances (protein amino acids, carbohydrates, fatty acids). Typically, the disease manifests in neonatal age within a few hours or days, an unbalanced concentration of toxic molecules occurs after increased intake of food in the diet or fever infections, as an acute condition with a change in behavior to coma (eg. hypoketotic coma in MCAD deficiency). Attacks can occur repeatedly, in conjunction with the specific situation the patient associates with (long starvation or vice versa sudden overeating).
However, some diseases may differ from this pattern and have a subacute or chronic form and affect organs other than the CNS.
Diseases of complex molecules
Diseases of large molecules are caused by a defect in the metabolism (disorder of production, transport of substances, but also in their degradation) of endogenously formed macromolecules (glycosaminoglycans, glycolipids, glycoproteins and others). Some of these substances form the building blocks of cell membranes, which then results in a defect of this type, others are broken down in proxisomes and lysosomes, in which they can then accumulate. It lasts for months to years, the disease runs without attacks and obvious short-term nutritional or infectious connections, it has a chronic charcter, which manifests itself only after the latent phase, during which enough macromolecules have accumulated for the defect to manifest itself at the level of function.
Diseases in which macromolecules accumulate in peroxisomes or lysosomes can mimic neurodegenerative or cancerous diseases. Diseases in which membrane defects occur include chromosomal aberrations, such as organomegaly, head and face dysmorphia, CNS and other organ involvement.
Therapy
Reduction of substrate intake by diet during substrate accumulation (diet), supplementation with food or parenterally in case of lack of product (diet), enzymatic treatment of peroxisomal diseases, influence of metabolic pathway (inhibition, formation of by-products - eg. administration of cysteamine in cystinosis), organ transplantation and theoretically gene therapy.
Links
Source
Materials used with kind permission of doc. MUDr. Viktora Kožicha, CSc.
comment
- ↑ Prevalence chorob, pro něž je v USA prováděn novorozenecký screening, se uvádí přibližně mezi 1:900–1:2000 Template:Doplňte zdroj
- ↑ Předběžná data z projektu Region 4 Genetics, [1]
Reference
References
- KOŽICH, Viktor: Úvod do biochemické genetiky dědičných metabolických poruch. [přednáška z patobiochemie], 5. 10. 2010
- KOŽICH, Viktor a Jiří ZEMAN. Dědičné metabolické poruchy v pediatrii. Postgraduální medicína [online]. 2010, roč. 12, vol. 7, s. 793–799, dostupné také z <https://zdravi.euro.cz/>. ISSN 1214-7664.
- FERNANDES, John, Jean-Marie SAUDUBRAY a Georges van den BERGHE, et al. Diagnostika a léčba dědičných metabolických poruch. 4. vydání. Praha : Triton, 2008. 607 s. ISBN 978-80-7387-096-6.
Template:Šablona:Navbox - dědičné metabolické poruchy
Kategorie:Patobiochemie Kategorie:Genetika Kategorie:Pediatrie