Glycemia/determination
< Glycemia
Determination of the concentration of glucose in the blood is an examination that will provide basic information about carbohydrate metabolism. Capillary or venous blood is collected and glucose is determined in whole blood, plasma or serum . When determining glucose in whole blood, the values are 10-15% lower (depending on the hematocrit ), in arterial blood they are 10% higher than in venous (arteriovenous difference). To prevent glycolysis, NaF (2.5 mg per 1 ml of whole blood) is added to the collection containers.
Examination of the concentration of glucose in the blood has the necessary informational value only if the time interval between blood sampling and food intake is known.
A blood glucose test is performed:
- on an empty stomach (blood is taken at least 8 hours after eating) - indicated when searching for diabetics and determining the diagnosis of DM ;
- randomly measured blood glucose (blood is taken without indicating the temporal relationship to food intake) - performed when hypoglycemia or hyperglycemia is suspected;
- after a meal - postprandial glycemia (1 hour after a meal containing carbohydrates ) - indicated when checking the effectiveness of DM treatment;
- as a glycemic profile – blood glucose is determined several times a day, usually before main meals, sometimes after meals and at night.
Methods of determination of glycemia[edit | edit source]
Determination of glycemia in laboratory conditions[edit | edit source]
Various methods are used to determine glucose concentration. Enzyme methods are widespread . Glucose can be determined using any enzyme that metabolizes it. The possibilities of non-invasive blood glucose measurement are discussed in another article
Glucose oxidase reaction[edit | edit source]
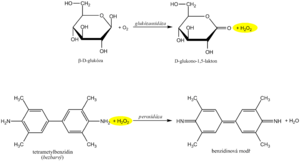
The recommended routine method uses the coupled enzyme reactions of glucose oxidase ( GOD , EC 1.1.3.4 ) and peroxidase ( POD , EC 1.11.1.7 ) . In the first reaction, the enzyme glucose oxidase catalyzes the oxidation of glucose by atmospheric oxygen to form gluconolactone. It is known that 36% of glucose in solution is in the form of the α-anomer and 64% in the form of the β-anomer. GOD is highly specific for β-D-glucopyranose. In order to oxidize both anomers, α- to β-anomer mutarotation is necessary, which occurs spontaneously during a sufficiently long incubation. An equimolar amount of hydrogen peroxide is produced as a byproduct of the glucose oxidase reaction .
In another reaction catalyzed by peroxidase , the resulting hydrogen peroxide reacts with a suitable chromogen, which is oxidized to a reactive intermediate, which couples with another substance to a permanent soluble dye. An example can be the oxidative coupling of a phenol derivative with 4-aminoantipyrine to a red dye, the absorbance of which is measured after the reaction equilibrium is established.
Other methods use the measurement of oxygen loss , which occurs during the reaction catalyzed by glucose oxidase and which we can monitor electrochemically with an oxygen electrode or an enzyme electrode.
Hexokinase reaction[edit | edit source]
The hexokinase method is characterized by high specificity. Hexokinase ( EC 2.7.1.1 ) phosphorylates glucose in the presence of ATP to glucose-6-phosphate . In the next step, glucose-6-phosphate is oxidized by glucose-6-phosphate dehydrogenase against NADP + to 6-phosphogluconolactone. The reduction of NADP + to NADPH can be evaluated by direct photometry in the UV region based on the principle of the Warburg optical test .
Determination of glycemia in non-laboratory conditions[edit | edit source]
Glycemia is among the parameters that are often examined even without a laboratory background. Rapid blood glucose determination is common in emergency care. In patients treated with insulin, blood sugar levels are preferably monitored regularly using a personal glucometer , and the treatment is adjusted based on the measured values. The concentration of glucose in the blood is among the parameters most often determined by examination techniques at the point of care for the patient ( point of care testing , POCT ). However, it must be kept in mind that POCT methods, as much as they improve the quality of care and the comfort of the patient, do not replace regular medical examinations or laboratory tests.
Methods of rapid blood glucose determination use several principles. The starting material is usually a drop of whole capillary blood that is applied to a test strip .
The earliest strips were based on the same reactions as the photometric measurement of glucose concentration. The reaction zone contained glucose oxidase , peroxidase and the appropriate chromogen. The evaluation was carried out either visually by comparison with a color scale, or using a glucometer - a single-purpose reflective photometer .
Most glucometers today use enzyme electrodes .
The first generation sensors appeared already in the sixties of the 20th century. The oldest system was based on the glucose oxidase reaction. He used two electrodes, one was covered with an enzyme. The concentration of oxygen in the sample and the rate of its decrease during the reaction was measured by the so-called Clark method: oxygen is reduced on the platinum cathode, the intensity of the current between the cathode and the anode corresponds to its concentration:
- O2 + 4 H+ + 4 e- → 2 H2O
Later, hydrogen peroxide production was determined electrochemically instead of oxygen consumption. In this case too, it is a simple electrochemical reaction, this time taking place at the anode:
- H2O2 → O2 + 2 H+ + 2 e-
The analyzers constructed in this way were simpler and could be more miniaturized. However, the amperometric measurement of hydrogen peroxide conductance is affected by a number of substances: ascorbate, uric acid, many drugs, etc. Another problem with many first-generation sensors was the dependence of the measurement results on the saturation of the sample with oxygen.
The sensors of the second generation are also based on the glucose oxidase reaction, but instead of molecular oxygen, the electron acceptor is another substance - the so-called mediator . Another possibility is the oxidation of glucose to gluconolactone by another bacterial enzyme, glucose dehydrogenase , with the electrons again being transferred to a suitable mediator. In both cases, the reduced mediator is oxidized again at the anode, and either the current flowing between the cathode and the anode (amperometric determination) or the resulting anode charge (coulombometric determination) is measured. A number of specific arrangements of test strips are used, various substances are used as mediators (e.g. cyanoferricate, ruthenium hexamine, osmium complexes, phenanthrolinequinone).
Links[edit | edit source]
References[edit | edit source]
- JOSEPH, Wang. Electrochemical glucose biosensors. Chemical reviews [online] . 2008, vol. 108, no. 2, pp. 814-825, also available from < https://pubs.acs.org/action/cookieAbsent >. ISSN 0009-2665.
- ↑ HELLER, Adam and Ben FELDMAN. Electrochemical glucose sensors and their applications in diabetes management. Chemical reviews [online] . 2008, vol. 108, no. 7, pp. 2482-2505, also available from < https://pubs.acs.org/action/cookieAbsent >. ISSN 0009-2665.