Embryonic septation of the heart
Septation of the atrioventricular canal, primordial atrium, and ventricle begins around the middle of the fourth week and is essentially complete by the end of the fifth week. Although they will be described here separately, all of these processes occur simultaneously.
Septation of the atrioventricular canal[edit | edit source]
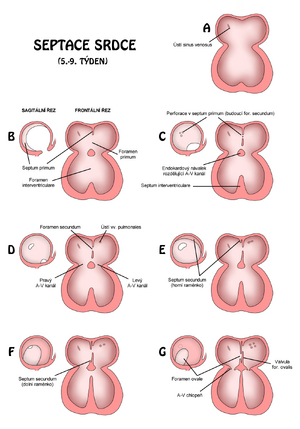
By the end of the fourth week endocardial cushions form on the dorsal and ventral walls of the atrioventricular (AV) canal. With the invasion of mesenchymal cells in the sixth week, the pads come closer together and fuse. It thus divides the AV canal into right and left, and also partially separates the primitive atria from the ventricular space.
- The endocardial cushions perform the function of the AV valves
Septation of the primitive atrium[edit | edit source]
With the formation and subsequent fusion of two septa, septum primum a septum secundum, the primitive atrium s divided into right and left atrium.
- Septum primum
- The septum primum grows from the ceiling of the primitive atrium towards the endocardial cushions. This half-lunar membrane thus partially partitions the common atrium for both the left and right heart. Since the membrane does not reach the pads, an opening remains between them - the – foramen (ostium) primum. This hole serves as a shunt through which oxygenated blood flows from the right atrium straight to the left. Most of the blood thus "bypasses" lungs, which of course are not yet breathing, so they do not even oxygenate, but only consume oxygen.
- But it is not yet the final form of this short circuit. The foramen primum shrinks and disappears with the fusion of the septum primum with the AV cushions merging into the primitive AV septum. (see above)
- Before it disappears, small perforations appear in its central part, caused by the apoptosis of septum primum cells. They gradually merge into a larger opening, the foramen (ostium) secundum. This takes over the function of the foramen primum.
- Septum secundum
- The septum secundum is again crescent-shaped, but a muscular membrane. It arises from the ventrocranial wall of the atrium just next to and to the right of the septum primum. During the 5th-6th week gradually covers the entire ostium secundum. It creates an incomplete partition between the halls. The upper part of the septum primum disappears, the remaining part (attached to the endocardial cushions) functions as the valvula foraminas ovalis, i.e. a valve that prevents backflow through the foramen ovale.
Note After birth, the foramen ovale closes and the valvula foraminis ovalis fuses with the septum secundum. It will thus create a complete partition between the halls.
Septation of the primitive ventricle[edit | edit source]
More precisely, septation takes place between the bulb and the primary chamber. At first, the division is indicated by a muscular strip - the primitive interventricular septum (IV septum) - pars muscularis septi interventriculorum. Initially, its increase is caused by dilation of both neighboring structures. Later, the active proliferation of myoblasts of the septum, which gradually increases, also participates in this process. Until the seventh week, it persists between the free edge of the IV septum and the fused endocardial pads of the semilunar foramen interventriculare. This usually closes by the end of the seventh week when the bulbar laminae fuse with the endocardial cushions to form the pars membranacea septi interventriculorum The closure of foramen IV (= pars membranacea formation) is therefore the result of the fusion of tissues originating from three sources:
- right bulbar bars;
- left bulbar ridges;
- endocardial cushions.
After closing the foramen interventriculare and forming the membranous part of the IV septum, the truncus pulmonalis communicates with the right ventricle, and the aorta emerges from the left ventricle.
Septation of bulbus cordis and truncus arteriosus[edit | edit source]
Bulbus cordis is a structure that is later incorporated into the walls of the definitive ventricles. In the right ventricle, the conus arteriosus arises from the bulb , from which the truncus pulmonalis emerges. In the left ventricle, the bulbus cordis forms the walls of the vestibulum aortae , which is the part of the aorta just in front of the [aortic] valves.
The bulbus is divided into the truncus ( aorta and pulmonary trunk), the conus (outflow portion of the aorta and pulmonary trunk), and the trabecular portion of the right ventricle .
The truncal region is subsequently divided by the aorticopulmonary septum, this septum has a spiral shape and thus divides this region into two main arteries.
Progress[edit | edit source]
In the 5th week zproliferation of the mesenchyme in the walls of the bulb and the formation of bulbar ridges begin . These slats are followed by similar truncal ones . Both are derived from neural crest mesenchyme.
- Right upper truncal bar.
- Left lower truncal bar.
The slats grow against the aortic sac and wrap around each other, predicting the future spiral course of the septum. Thus, after the fusion, an aorticopulmonary septum is formed , which divides the truncus into the aorta and the pulmonary trunk.
Also, the bars in the conus cordis grow against each other and later join the truncal septum. After the fusion of the two slats in the conus region, the conus is divided into the outflow region of the right and left ventricles.
A very important role here is played by the migration . of neural crest cells, which contribute especially to the formation of endocardial crests in the area of the conus and truncus arteriosus Abnormal migration at this site gives rise to many congenital malformations.
- Tetralogy of Fallot, which includes pulmonary stenosis, persistence of the truncus arteriosus, and transposition of the great vessels.
Neural crest cells also contribute to the development of craniofacial structures, which is why we often see changes in the facial area with cardiac abnormalities.
Links[edit | edit source]
Related Articles[edit | edit source]
Sources[edit | edit source]
- MOORE, Keith L – PERSAUD,. Zrození života : Embryologie s klinickým zaměřením. české 1 edition. ISV nakladatelství, 2002. Chapter 5. ISBN 80-85866-94-3.
- SADLER, Tomas W. Langman’s medical embryology. 10. edition. Lippincott Williams & Wilkins, 2006. 371 pp. ISBN 0-7817-9485-4.