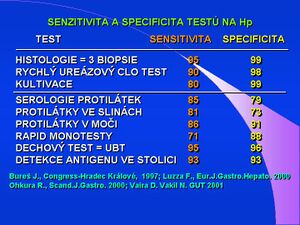
Determination of antibodies against Helicobacter pylori
Antibodies to Helicobacter pylori in serum can be detected by a whole range of immunological and serological techniques such as immunoblotting, immunofluorescence , hemagglutination , complement fixation, latex tests, etc. However, the most widespread method is undoubtedly ELISA - a simple, fast, cheap and reliable technique. However, the specificity and sensitivity of the method is highly dependent on the antigen used – from whole cells, via ultrasonic sonication, glycine extract to purified proteins. In 1989, the isolation of a high molecular weight surface protein (designated HM-CAP, high molecular weight cell-associated protein) was described, with a specificity and sensitivity of 95%.
Serological detection of antibodies to Helicobacter pylori is of clinical importance mainly for long-term follow-up after treatment and for monitoring the success of Helicobacter pylori eradication . A decrease in IgG after 6 months of treatment to values < 50% shows a specificity of 95% and a sensitivity of 97%. Indications include screening of high-risk patients, e.g. kidney transplant patients , when helicobacter infection increases the risk of peptic ulcer and bleeding.
Antibodies to Helicobacter pylori can also be detected in saliva or urine using immunological techniques. There are also a number of so-called rapid tests , where antibodies to Helicobacter pylori are detected from whole blood, after capillary sampling within a few minutes using the immunoaffinity chromatography method. The specificity and sensitivity of these tests is relatively low – 70 to 85%.
The serological diagnosis of antibodies to Helicobacter pylori also includes the detection of cagA, vacA and iceA antigens, the presence of which specifies strains of Helicobacter pylori with higher pathogenicity. Classic ELISA tests on microtitre plates or PCR techniques are used to detect these antigens. Detection of Helicobacter pylori and its strains by PCR methods is in the phase of clinical testing, it is not yet used in routine diagnostics. The diagnostic value of determining antibodies to Helicobacter pylori is still a subject of research, it is not suitable for screening programs.
Links[edit | edit source]
Related Articles[edit | edit source]
- diagnosis of helicobacter infection
- Breath tests with carbon-13
- Detection of Helicobacter pylori antigen in stool
Source[edit | edit source]
- KOCNA, Petr. GastroLab [online]. [cit. 2011-03-04]. <http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm>.
References[edit | edit source]
- PENG, NJ. Comparison of noninvasive diagnostic tests for Helicobacter pylori infection. Medical Principles and Practice (International Journal of the Kuwait University Health Sciences Centre) [online]. 2009, vol. 18, no. 1, p. 57-61, Available from <https://www.ncbi.nlm.nih.gov/pubmed/19060493?ordinalpos=19&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum>. ISSN 1011-7571, eISSN 1423-0151. PMID: 19060493.
- JANULAITYTE-GÜNTHER, D. Combined serum IgG response to Helicobacter pylori VacA and CagA predicts gastric cancer. FEMS immunology and medical microbiology [online]. 2007, vol. 50, no. 2, p. 220-225, Available from <https://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17567283&ordinalpos=31&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum>. ISSN 0928-8244, eISSN 1574-695X. PMID: 17567283.
- ZAMBON, CF. Non-invasive diagnosis of Helicobacter pylori infection: simplified 13C-urea breath test, stool antigen testing, or DNA PCR in human feces in a clinical laboratory setting?. Clinical biochemistry [online]. 2004, vol. 37, no. 4, p. 261-267, Available from <https://www.ncbi.nlm.nih.gov/pubmed/15003727?dopt=Abstract>. ISSN 0009-9120, eISSN 1873-2933. PMID: 15003727.
- CHEN, TS. Immunoglobulin G antibody against Helicobacter pylori: clinical implications of levels found in serum. Clinical and diagnostic laboratory immunology [online]. 2002, vol. 9, no. 5, p. 1044-1048, Available from <https://www.ncbi.nlm.nih.gov/pubmed/12204957?dopt=Abstract>. ISSN 1071-412X, eISSN 1098-6588. PMID: 12204957.
- KINDERMANN, A. Evaluation of a rapid whole blood test to detect Helicobacter pylori infection in children. Scandinavian journal of gastroenterology [online]. 2001, vol. 36, no. 6, p. 572-576, Available from <https://www.ncbi.nlm.nih.gov/pubmed/11424314?dopt=Abstract>. ISSN 0036-5521, eISSN 1502-7708. PMID: 11424314.
- ARENTS, NL. The importance of vacA, cagA, and iceA genotypes of Helicobacter pylori infection in peptic ulcer disease and gastroesophageal reflux disease. The American journal of gastroenterology [online]. 2002, vol. 97, no. 4, p. 1065, Available from <https://www.ncbi.nlm.nih.gov/pubmed/11569682?dopt=Abstract>. ISSN 0002-9270, eISSN 1572-0241. PMID: 11569682.