DNA isolation and genetic markers
The development of molecular biology and the application of PCR in routine diagnostics are opening up completely new trends in the screening of GIT tumors. The latest screening methods are based on the detection of specific mutations by PCR or biochip technology in DNA isolated from stool samples.
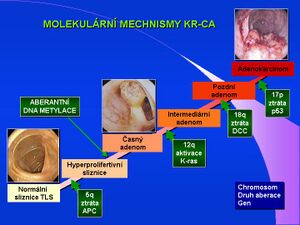
Molecular biology offers the possibility to detect individual genetic markers of the colorectal cancer process in the adenoma-carcinoma sequence: loss/mutation of APC gene at 5q, overexpression of COX-2, activation/mutation of K-ras at 12q, loss/mutation of p53 at 17p, loss of DCC at 18q. The gene mutations can be detected in a biopsy sample of colon tissue or in a stool sample after DNA isolation from colonic mucosal epithelia. Commercially available kits provide isolation of 10-30 μg DNA from a 220 mg stool sample in a 50-minute process and removal of inhibitors for further PCR analysis. Real-time PCR techniques allow, for example, the detection of hypermethylation of the SFRP2 gene in DNA isolated from stool as a marker of colorectal cancer.
Links[edit | edit source]
Related articles[edit | edit source]
Source[edit | edit source]
- taken with permission of the author from KOCNA, Petr. GastroLab : MiniEncyklopedie laboratorních metod v gastroenterologii [online]. ©2002. Poslední revize 2011-01-08, [cit. 2011-03-04]. <http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm>.
Reference[edit | edit source]
- CALISTRI, D, et al. Quantitative fluorescence determination of long-fragment DNA in stool as a marker for the early detection of colorectal cancer. Cell Oncol. 2009, vol. 31, no. 1, s. 11-7, ISSN 1570-5870 (Print), 1875-8606 (Electronic). PMID: 19096146.
- KOGA, Y, et al. Detection of the DNA point mutation of colorectal cancer cells isolated from feces stored under different conditions. Jpn J Clin Oncol. 2009, vol. 39, no. 1, s. 62-9, ISSN 0368-2811 (Print), 1465-3621 (Electronic). PMID: 19042945.
- AHLQUIST, DA, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008, vol. 149, no. 7, s. 441-50, ISSN 0003-4819 (Print), 1539-3704 (Electronic). PMID: 18838724.
- ITZKOWITZ, S, et al. A simplified, noninvasive stool DNA test for colorectal cancer detection. Am J Gastroenterol. 2008, vol. 103, no. 11, s. 2862-70, ISSN 0002-9270 (Print), 1572-0241 (Electronic). PMID: 18759824.
- WANG, DR, et al. Hypermethylated SFRP2 gene in fecal DNA is a high potential biomarker for colorectal cancer noninvasive screening. World J Gastroenterol. 2008, vol. 14, no. 4, s. 524-31, ISSN 1007-9327. PMID: 18203283.
- RENNERT, G, et al. Detecting K-ras mutations in stool from fecal occult blood test cards in multiphasic screening for colorectal cancer. Cancer Lett. 2007, vol. 253, no. 2, s. 258-64, ISSN 0304-3835 (Print), 1872-7980 (Electronic). PMID: 17349741.
- HAUG, U, et al. Mutant-enriched PCR and allele-specific hybridization reaction to detect K-ras mutations in stool DNA: high prevalence in a large sample of older adults. Clin Chem. 2007, vol. 53, no. 4, s. 787-90, ISSN : 0009-9147 (Print), 1530-8561 (Electronic). PMID: 17317884.
- MATSUSHITA, H, et al. A new method for isolating colonocytes from naturally evacuated feces and its clinical application to colorectal cancer diagnosis. Gastroenterology. 2005, vol. 129, no. 6, s. 1918-27, ISSN 0016-5085 (Print), 1528-0012 (Electronic). PMID: 16344060.
- GREENWALD, B. The stool DNA test: an emerging technology in colorectal cancer screening. Gastroenterol Nurs. 2005, vol. 28, no. 1, s. 28-32, ISSN 1042-895X (Print), 1538-9766 (Electronic). PMID: 15738729.
- OUYANG, DL. Noninvasive testing for colorectal cancer: a review. Am J Gastroenterol. 2005, vol. 100, no. 6, s. 1393-403, ISSN 0002-9270 (Print), 1572-0241 (Electronic). PMID: 15929776.
- WHITNEY, D, et al. Enhanced retrieval of DNA from human fecal samples results in improved performance of colorectal cancer screening test. J Mol Diagn. 2004, vol. 6, no. 4, s. 386-95, ISSN 1525-1578 (Print), 1943-7811 (Electronic). PMID: 15507679.
- IMPERIALE, TF, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004, vol. 351, no. 26, s. 2704-14, ISSN 0028-4793 (Print), 1533-4406 (Electronic). PMID: 15616205.
- BRAND, RE, et al. Reproducibility of a multitarget stool-based DNA assay for colorectal cancer detection. Am J Gastroenterol. 2004, vol. 99, no. 7, s. 1338-41, ISSN 0002-9270 (Print), 1572-0241 (Electronic). PMID: 15233675.
- SONG, K, et al. Fecal DNA testing compared with conventional colorectal cancer screening methods: a decision analysis. Gastroenterology. 2004, vol. 126, no. 5, s. 1270-9, ISSN 0016-5085 (Print), 1528-0012 (Electronic). PMID: 15131787.
- LEVIN, B, et al. Emerging technologies in screening for colorectal cancer: CT colonography, immunochemical fecal occult blood tests, and stool screening using molecular markers. CA Cancers J Clin. 2003, vol. 53, no. 1, s. 44-55, ISSN 0007-9235 (Print), 1542-4863 (Electronic). PMID: 12568443.
- NISHIKAWA, T, et al. A simple method of detecting K-ras point mutations in stool samples for colorectal cancer screening using one-step polymerase chain reaction/restriction fragment length polymorphism analysis. Clin Chim Acta. 2002, vol. 318, no. 1-2, s. 107-12, ISSN 0009-8981 (Print), 1873-3492 (Electronic). PMID: 11880119.
- PRIX, L, et al. Diagnostic biochip array for fast and sensitive detection of K-ras mutations in stool. Clin Chem. 2002, vol. 43, no. 3, s. 428-35, ISSN 0009-9147 (Print), 1530-8561 (Electronic). PMID: 11861435.