Colorectal carcinoma/screening
As in the case of all other malignancies occurring widely in the population, efforts have been made in the case of colorectal cancer (CR-CA) to develop effective screening for early stages of the disease as part of secondary prevention. Ideally, precancerous lesions (adenomas). One of the main problems of the still rather high mortality of CR-CA is that in most cases it is diagnosed only at a very advanced stage. CR-CA can now be described as one of the three malignancies for which we have comprehensive screening (breast cancer and cervical cancer), which effectively reduces morbidity and mortality of this disease in the population.
- Screening methods
The basic screening methods of CR-CA in the Czech republic include:
- Fecal occult blood test (FOBT) – according to four randomized trials, the introduction of FOBT has reduced CR-CA mortality in people aged 50-80 years by 15-33% [1]
- Primary screening colonoscopy - also demonstrably reduces the risk of CR-CA mortality [1]
- Screening procedure
CR-CA screening is performed and covered by the insurance company for asymptomatic men and women over the age of 50. However, all high-risk patients with a positive personal or family history are excluded from the screening program, and special dispensary programs are developed for these individuals, depending on their risk.
Individuals within the CR-CA screening are divided into two groups according to age, and the examination procedure differs within these two groups:
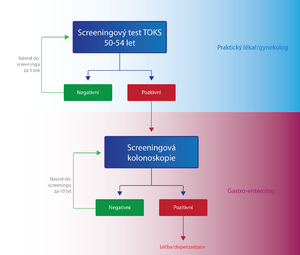
- Persons aged 50-54
- Once a year, the patient is performed either by a general practitioner or a FOBT gynecologist. If the test result is negative (FOBT-), the same test is performed on the patient again after one year. In case of a positive result (FOBT+), the patient is sent to a specialized facility for screening colonoscopy . If the finding on a colonoscopy is negative, the patient will come for another screening examination after 10 years, if it is positive, the patient is included in the high-risk group with a special dispensary program in terms of CR-CA screening. [2]
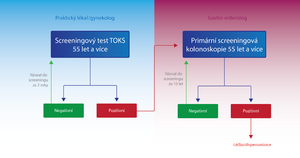
- Persons over 55 years
- In addition to FOBT, primary screening colonoscopy is used for these individuals. The patient has a choice.
- FOBT - in the case of FOBT- either the test is repeated after two years or a primary screening colonoscopy is performed. In the case of FOBT+, the patient is sent for screening colonoscopy as in the previous group. [2]
- Primary screening colonoscopy - is an alternative method to FOBT. In the case of a negative result, it is performed again in 10 years; in the case of a positive result, the patient is included in the high-risk group with a special dispensary program in terms of CR-CA screening. [2]
The importance of colonoscopy in screening is likely to increase with the development of non-invasive virtual colonoscopy methods. [1]
Molecular genetic screening techniques, which have significantly higher sensitivity, are being introduced into practice. They are based on the detection of mutations and aberrant methylations typical of adenocarcinoma cells or advanced adenoma[3].
Links[edit | edit source]
Source[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
References[edit | edit source]
- ↑ a b c ČEŠKA, Richard. Interna. 1. edition. Praha : Triton, 2010. 855 pp. pp. 412. ISBN 978-80-7387-423-0.
- ↑ a b c DUŠEK, L. Kolorektum.cz – Program kolorektálního screeningu v České republice [online]. Kolorektum.cz, ©2015. The last revision 23. 2. 2015, [cit. 2015-11-11]. <http://www.kolorektum.cz/index.php?pg=pro-odborniky--organizace--screeningovy-proces>.
- ↑ IMPERIALE, Thomas F – RANSOHOFF, David F – ITZKOWITZ, Steven H. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med [online]. 2014, vol. 371, no. 2, p. 187-188, Available from <https://www.ncbi.nlm.nih.gov/pubmed/2500673>. ISSN 0028-4793 (print), 1533-4406.
References used[edit | edit source]
- ČEŠKA, Richard. Interna. 1. edition. Praha : Triton, 2010. 855 pp. pp. 412. ISBN 978-80-7387-423-0.
Recommended literature[edit | edit source]
- PETRUŽELKA, Luboš – KONOPÍSEK, Bohuslav. Klinická onkologie. 1. edition. Praha : Karolinum, 2003. 274 pp. ISBN 80-246-0395-0.
- KRŠKA, Zdeněk – HOSKOVEC, David. Chirurgická onkologie. 1. edition. Praha : Grada, 2014. 904 pp. ISBN 978-80-247-4284-7.