Blood drawing
Peripheral venous blood of adults is most often collected using closed collection systems. The person collecting blood is protected from contamination with the patient's blood and at the same time external contamination of the collected biological material is prevented. Mainly vacuum collection tubes are used. The disposable collection tube already contains preparation agents (e.g. anticoagulants or, conversely, coagulation accelerators). It is hermetically sealed and a vacuum is created in it, which ensures that the right amount of blood is sucked in during sampling. This also ensures the correct ratio of blood and preparation reagents.
Collection tubes and material prepared from venous blood[edit | edit source]
Serum[edit | edit source]
Blood serum is the basic material for most routine clinical-biochemical determinations. It is prepared from collected whole venous blood by its precipitation and centrifugation. This will get rid of blood elements, fibrinogen and most other coagulation factors from the material. Precipitation of fibrin reduces the total protein concentration in serum by about 4 g/l compared to plasma. The concentration of calcium, which is consumed during coagulation, also decreases slightly.
The advantage of serum is the good stability of most analytes (usually hours to days when stored in the cold), it is often even possible to freeze the sample and store it for a long time. The disadvantage is the longer preparation of the material, coagulation and centrifugation takes around 45 minutes.
Blood for obtaining serum can be collected in a test tube without any reagents, coagulation occurs after contact with glass. Precipitation, however, takes quite a long time, for reliable results you need to wait at least 60 minutes before centrifugation. This increases the risk of hemolysis and release of intracellular components from blood elements. At the same time, metabolism in blood cells can continue for some time, which also affects the concentration of some substances. That is why tubes are mainly used, in which coagulation activators have been added – e.g. different forms of silicon dioxide. The addition of the activator reduces the time required for reliable coagulum precipitation to 30 minutes.
For easier separation of the serum, there may be a separation gel in the collection tube. During centrifugation, blood elements and coagulum sediment under the gel, the serum remains above it. The gel facilitates the separation of the serum and at the same time it prevents contamination of the serum with the intracellular content of blood elements, which is gradually released. The separation gel is usually made of acrylate polymers with the addition of silicon dioxide, which further accelerates coagulation.
Collection tubes for the preparation of blood serum usually have a golden yellow (with separation gel) or a red (without gel) stopper.
Plasma and heparinized whole blood[edit | edit source]
The basic material for most emergency ('statim') biochemical tests is blood plasma. Lithium heparin is used as an anticoagulant for its preparation. The preparation of plasma is faster than the preparation of serum, because the time required for blood clotting is eliminated. The tubes have walls coated with dried heparin. After collection, the blood is mixed with it by repeatedly turning the test tube, and centrifugation can be started immediately afterwards. Tubes for plasma preparation may also contain a gel to facilitate the separation of plasma from blood elements.
The addition of heparin has relatively little effect on common biochemical determinations. The lithium salt of heparin is most often used because, unlike sodium or potassium heparin, it does not change the concentration of sodium or potassium, i.e. basic and commonly determined ions.
As an anticoagulant, heparin can also be used for some examinations of "whole blood", e.g. examination of acid-base balance and blood gases. In that case, heparinized capillaries or special sampling sets with a syringe for anaerobic sampling are used. However, heparinized blood is not suitable for hematological examinations (heparin interferes with the staining of blood smears) or for molecular biological methods (it inhibits the polymerases used in PCR).
Vacuum tubes with heparin are usually marked green – tubes with separation gel have a light green stopper, and tubes without the separation gel have a dark green stopper.
Whole blood with EDTA[edit | edit source]
For examination of blood elements (blood count) non-coagulable whole blood is used. One of the salts of ethylenediamine tetraacetic acid (EDTA) serves as an anticoagulant in this case. The most commonly used are well-soluble potassium salts, K2-EDTA and K3-EDTA. Whole blood with EDTA is also suitable for the examination of most analytes that are intracellular in blood cells, e.g. "glycated hemoglobin" and for "DNA analysis and molecular-biological methods".
Blood with the addition of EDTA is not suitable for many other biochemical methods. The concentration of ions changes significantly and a number of enzymes are inhibited. EDTA also cannot be used to test blood coagulation, although the mechanism of its anticoagulant effect is based on the chelation of calcium ions, similar to the effect of citrate. However, the effect of EDTA cannot be completely canceled by plasma recalcification, probably because EDTA also absorbs other metals (e.g. copper) that are necessary for the function of some coagulation factors (e.g. f. V and VIII).
Test tubes with the addition of EDTA salts are most often marked with a violet (lavender) stopper.
Citrated plasma and citrated whole blood[edit | edit source]
Examination of coagulation parameters is performed from plasma decalcified with citrate. Buffered solutions of sodium citrate are mainly used. Whole blood with citrate, possibly with other additives (theophylline, adenosine and dipyridamole) is used to determine platelet functions. Citrate is also used as an anticoagulant in measuring the rate of erythrocyte sedimentation.
Citrate changes the ion concentration and inhibits some enzymes. Therefore, citrate plasma is not suitable for most biochemical determinations.
Tubes with the addition of citrate are marked with a light blue stopper, special tubes for erythrocyte sedimentation have a black stopper.
Plasma, serum and whole blood with glycolysis inhibitor[edit | edit source]
Even after blood collection, blood cells consume glucose. The glucose concentration thus gradually decreases during sample processing, depending on the temperature and other factors, by about 0.5 mmol/l per hour. Therefore, for a more reliable blood glucose determination, sampling tubes with the addition of sodium fluoride, which inhibits glycolysis, are used. There are variants both with an anticoagulant (Na2EDTA or potassium oxalate) for obtaining plasma, and without anticoagulant for serum preparation.
Fluoride collection tubes have a grey stopper.
Other and special collection tubes[edit | edit source]
A number of other variants of sampling tubes are commercially available for various other purposes – e.g. for taking samples for microbiological cultures, for toxicological analyses, determination of trace elements, with peptidase inhibitors, etc.
Other equipment necessary for venous blood collection[edit | edit source]
The quality of the material is also affected by other aids and medical devices used for sampling. Their correct choice is therefore an important part of the pre-analytical phase of the examination.
Disinfectant[edit | edit source]
For skin disinfection, alcohol disinfectants are recommended, most often 70% 2-propanol. In addition to the requirements for antimicrobial efficiency, it is desirable from the point of view of the pre-analytical phase that the disinfectant evaporates quickly. Contamination of the collected blood with alcohol leads to hemolysis and thus to the deterioration of the sample for a number of determinations.
Iodine preparations are less suitable for blood sampling, because if they even minimally contaminate the sample, they can affect a number of determinations.
Injection needles[edit | edit source]
With closed vacuum sampling systems, special double-sided needles are most often used. When assembling the system, the cover (usually white or gray) on the side facing the tube is removed from the needle. This end of the needle is also protected by a rubber sleeve to avoid injury during handling. The needle is screwed into the holder. Just before sampling, the second cover is removed (the color corresponds to the diameter of the needle). This coloured cover protects the tip, with which the vein will be punctured. Finally, after inserting the needle into the vein, the sampling tube is inserted into the holder, while its cap is pierced with the tip, still covered by the rubber sleeve.
The blood collection needle must have a suitable diameter. Using too thin a needle with a vacuum system will cause large pressure differences, which can lead to hemolysis. At the same time, the collection period will be extended. If sampling is slow, initially there is a high concentration of reagents in the tube, which again can lead to hemolysis. On the other hand, a needle that is too thick will cause a larger wound, the collection may be more painful, and smaller veins may be damaged.
The diameter of injection needles is traditionally expressed in the so-called Birmingham gauge, and is marked with the letter G. A higher number means a thinner needle. 23 G to 18 G needles are used for blood sampling.
| Color | Birmingham gauge |
Outer diameter (mm) |
Use |
|---|---|---|---|
| 23 G | 0.64 | In pediatrics | |
| 22 G | 0.72 | ||
| 21 G | 0.82 | Most often in adults | |
| 20 G | 0.91 | ||
| 18 G | 1.27 | Long collection – donating blood |
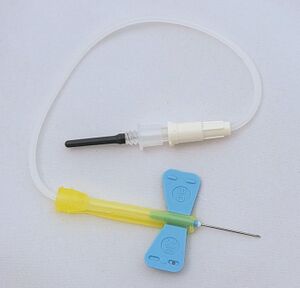
Sometimes it is more convenient to use a winged needle (butterfly) instead of a needle firmly attached to the holder. It is connected to the test tube holder by a flexible tube. It has flexible plastic "wings" on it to hold it while inserting it into a vein. A winged needle is a suitable alternative for more difficult situations. It is also more convenient when drawing large numbers of tubes and is safer when drawing blood from restless and uncooperative patients.
Some needles are equipped with a safety system that covers the tip after collection to minimize the risk of injury.
Tourniquet[edit | edit source]
In general, a tourniquet should be applied at the limb from which blood is taken for as short a time as possible, otherwise a number of measured values may change. If the tourniquet is applied for a long time, the risk of hemolysis of the sample also increases. Collection should take place within one minute after the tourniqut has been applied. The tourniquet must therefore be easy to put on and release again.
Disposable tourniquets are currently preferred to reduce the risk of patient-to-patient transmission. It is necessary to monitor what material the tourniquet is made of, especially due to allergies to latex.
Venous blood sampling procedure[edit | edit source]
- Prepare the tools:
- sampling tubes, needle and holder, tube stand,
- tourniquet, disinfectant, tampons or tissue wipes, patch bandage,
- containers for infectious and sharp waste,
- non-sterile gloves,
- blood test request forms, stationery,
- sampling tubes with affixed identification labels,
- bags for transporting biological material.
- Perform hygienic hand disinfection.
- Prepare and identify the patient.
- Introduce yourself and explain what you will be doing. Make sure the patient consents to the collection.
- A significant part of pre-analytical errors is caused by swapping samples. To avoid it, always just before blood drawing:
- ask the patient to say their full name' and/or check their name on the ID bracelet,
- check some additional data - e.g. hospital bed number, date of birth, name of referring doctor, residential address,
- similarly check request forms,
- check the identification labels on the tubes.
- Find out if the patient is allergic to anything (especially disinfectants, latex, patch).
- Find out if the patient has had an adverse reaction' to a blood draw (e.g. syncope) in the past. Assess unusual anxiosity.
- Ensure the patient is in a safe and comfortable sitting or supine position for sampling. The limb from which you will draw blood must be easily accessible to you and you must have all the tools within easy reach.
- Place a tissue pad under the limb from which you will draw blood.
- Find a location from which to collect. The vein should be visible and palpable even before the tourniquet is applied. It is not advisable to puncture the veins at the point of their branching, as this increases the risk of hematoma.
- Select the appropriate needle size. Place the needle in the holder.
- Disinfect the site of puncture.
- Disinfection is carried out from the center to the sides. Depending on the disinfectant used, it should take around 30 seconds, after which the disinfectant should dry completely (another 30 seconds or so). After disinfection, do not touch the site of puncture, otherwise the disinfection must be repeated.
- Perform hygienic hand disinfection' and put on gloves'.
- Place the tourniquet about 8–10 cm above the chosen place.
- Insert the needle into the vein.
- Fix the vein with your thumb below the injection site.
- Ask the patient to clench fist'.
- Insert the needle with a quick movement at an angle of about 30°.
- Fill the tubes
- Insert the test tube into the holder and pierce its stopper with a needle. The tube begins to fill with blood.
- Now ask the patient to relax the fist. Release the tourniquet.
- Fill all the prepared tubes one by one. According to WHO recommendations, the tubes are taken in the order coagulation (citrate, light blue) – serum (coagulation activator, golden yellow) – plasma (heparin, green) – blood count (EDTA, purple) – glucose (fluoride, gray).
- Let each test tube be filled to the prescribed volume (blood will stop flowing). The tubes usually have the blood level marked on the label.
- Once the filled tube is removed from the holder, gently mix it by turning it upside down and back. Tubes for the preparation of serum (golden yellow, red) should be turned 5× back and forth, others 8×.
- After filling the tubes, place dry tissue wipe or a tampon on the needle and pull the needle off the vein. Dispose the needle in a sharps container. Compress the injection site.
- Ask the patient to continue compressing the injection site. The limb should not be bent at the elbow, this increases the risk of a hematoma. Monitor the patient's condition after collection. Apply a patch after the bleeding has stopped.
- Again check the labels on the tubes and request forms. Place the test tubes and request forms in the transport bag.
- Decontaminate surfaces, remove gloves and perform hygienic hand disinfection.
Order of sampling tubes[edit | edit source]
When inserting a vacuumed sampling tube into the holder and piercing its stopper, the sampling needle may be contaminated by traces of the reagent on the inside of the stopper. Even this trace contamination can affect the results of some determinations. The tubes are therefore taken in an order that minimizes the occurrence of errors.
The tubes without additives are filled first, if they are used, or samples that require special collection purity (e.g. dark blue tubes for the determination of trace elements). Citrate plasma (light blue plug) for determination of coagulation parameters and citrate blood (black plug) for erythrocyte sedimentation follow. Even minimal contamination with a coagulation activator or, conversely, anticoagulant agents has a non-negligible effect on the determination of coagulation parameters and sedimentation.
Other tubes follow in the order of serum with coagulation activators (golden yellow or red), plasma with lithium heparin (green), whole blood for blood count with EDTA (violet) and other additives (e.g. gray tubes with fluoride for glucose determination).
| Coagulation parameters | Serum | Plasma (statim) | Blood count | Glucose |
| Citrate | Clotting activators | Lithium heparin | EDTA (potassium salts) | Fluoride |
Hemolysis of blood sample[edit | edit source]
A relatively common problem is hemolysis of blood during its pre-analytical processing. During hemolysis, intracellular components are released from blood cells and a number of results are distorted. It is impossible to determine, for example, serum concentrations of potassium, bilirubin, AST or lactate dehydrogenase activity, and many other determinations are imprecise.
Hemolysis can also occur intravascularly as a result of some pathological conditions. More often, however, it occurs during or after blood collection and is the result of incorrect collection technique. Causes may include:
- Contamination of the sample by disinfection during collection.
- Use alcohol disinfectant, let the disinfectant dry.
- Applying the tourniquet too long, exercising the limb before sampling.
- The tourniquet should be tightened for no longer than one minute.
- Large pressure differences during sampling.
- Use a stronger needle.
- Blood collection from hematoma.
- Avoid excessive trauma and vessel damage during collection.
- Shaking the sample after collection.
- Mixing the sample is done by slowly rotating the tube. Mechanical damage can also occur during transport by pipe mail.
- Insufficient mixing of the sample with the reagents in the tube.
- The tube must be mixed immediately after collection. Follow the recommended number of turns of the tube.
- Rapid temperature changes.
- Avoid rapid cooling of the sample after collection or freezing during transport.
Links[edit | edit source]
External Resources[edit | edit source]
Used Literature[edit | edit source]
- DHINGRA, Neelam. WHO Guidelines on Drawing Blood : Best Practices in Phlebotomy. 1. edition. WHO, 2010. 109 pp. ISBN 9789241599221.
- ZIMA, Tomáš. Laboratorní diagnostika. 3. edition. Galén, 2013. 1146 pp. ISBN 9788074920622.
- RACEK, Jaroslav. Klinická biochemie. 2. edition. Galén, 2006. 329 pp. ISBN 9788072623242.
- TIETZ, Norbert. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4. edition. Elsevier Saunders, 2006. 2412 pp. ISBN 9780721601892.