Biotransformation
Biotransformation is the transformation of the chemical structure of a substance by the action of a living organism. It is catalysed by [[enzymes]. The biological activity of a given substance can be reduced, unchanged or increased by biotransformation. An increase in biological activity can be therapeutically positive (e.g. the activation of a prodrug), or negative (e.g. the formation of a toxic metabolite).
Effect of polarity and ionisability of substances[edit | edit source]
- Hydrophilic, polar or ionisable substances are largely excreted by the kidneys in their original form.
- Lipophilic substances undergo extensive metabolism:
- They are reabsorbed from the renal glomerular filtrate into the blood and transformed into more polar metabolites so that these can be excreted in the urine.
- Lipophilic substances easily undergo enterohepatic metabolism and are partially excreted in the faeces.
Biotransformation reactions and their classification[edit | edit source]
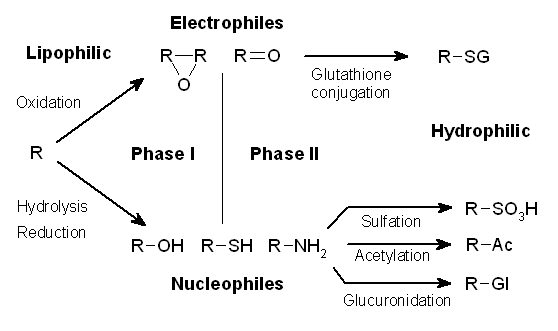
Usually, the biotransformation reaction is divided into two phases, named differently according to the personal preferences of the author of that publication. One possibility is the division into a non-synthetic phase and synthetic phase , which adequately and graphically describes the differences between these groups of biotransformation reactions. Alternatively, the first and second phase can be used to describe biotransformation, but the problem with this division is the fact that not every substance subject to biotransformation goes through both phases.
Phase I reaction, non-synthetic[edit | edit source]
Phase I reactions are oxidation reactions, and to a lesser extent reduction and hydrolytic reactions. During the non-synthetic phase, changes occur in the molecule of the transformed substance. Usually, functional groups are formed that enable a subsequent increase in hydrophilicity in the synthetic phase of biotransformation. The biological activity of the substance is usually reduced, but it can also be increased (e.g. enalapril is activated to enalaprilat, codeine is changed to morphine) or unchanged (diazepam is changed to nordiazepam). Toxic metabolites can also be formed in this phase (e.g. metabolites of halothane, paracetamol, and cyclophosphamide). Oxidation takes place most often, and reduction or hydrolytic reactions are less common.
An overview of the enzymatic systems involved in the non-synthetic phase:
- cytochrome P450 family oxidation system,
- CYP (family)(subfamily)(isoenzyme) is indicated below, e.g. CYP 3A4; 17 families are described,
- most drugs are metabolized by the CYP 3A4 and CYP 2D6 systems,
- microsomal oxidation system,
- mitochondrial amino oxidase,
- cytoplasmic alcohol and aldehyde dehydrogenase,
- xanthine oxidase,
- peroxidase,
- reductase (endoplasmic reticulum and cytoplasm),
- hydrolase (e.g. plasma esterase).
Phase II reaction, synthetic[edit | edit source]
In this phase of biotransformation, inactive hydrophilic compounds are usually formed, which can be easily excreted in the urine. The essence of this phase is the attachment of a hydrophilic functional group to the original substance. Depending on the nature of the substance and especially the attacked group, several reactions can take place:
- glucuronidation – conjugation of a ‑COOH or ‑OH group with glucuronic acid,
- acetylation – isoniazid, sulfonamides, aniline derivatives,
- polymorphism in acetylase makes treatment of tuberculosis with isoniazid problematic
- conjugation with glycine – acetylsalicylic acid, nicotinic acid,
- conjugation with glutathioneem – epoxides, nitro compounds, ethacrynic acid,
- formation of ethereal sulfates – minor pathway of phenolic compounds.
Induction of enzymatic activity[edit | edit source]
The activity of biotransformation enzymes increases due to the action of the xenobiotic. It serves as protection of the organism against chemical stress. It depends on the dose of the inducer. In particular, CYP oxidases can be induced, this phenomenon can be the basis of pharmacological tolerance.
Phenobarbital type of induction The activity of CYP 2B1, 2B2, 3A1, 3A4 and subfamily 2C increases in particular. The metabolism of phenobarbital (autoinduction) and other substances (heteroinduction), e.g. tolbutamide, cortisol and coumarin is increased. Liver mass also usually increases.
Benzpyrene type of induction The activity of CYP 1A1, 1A2, glutathione transferase, ALA synthase and other enzymes increases. In addition to benzpyrene, the metabolism of e.g. 3-methylcholanterene also increases. Liver mass increases only slightly.
Non-inducible enzymes Some enzymes cannot be induced. These include, for example, CYP 2D6.
Inhibition of enzyme activity[edit | edit source]
Reduction in the activity of biotransformation enzymes as a result of their interaction with the xenobiotic. their complete deactivation may occur. Symptoms of intoxication may appear several hours after administration of the substance. For example, fluoroquinolone completely inhibit CYP 3A4, quinidine heteroblocks CYP 2D6, and cimetidine and ketoconazole can block multiple enzymes by irreversibly binding to heme iron. Sometimes enzyme blockades are also used, e.g. xanthine oxidase allopurinolin gout therapy or aldehyde dehydrogenase blockade with disulfiramas part of alcohol addiction therapy.
Classification of enzymes according to the IUBMB (International Union of Biochemistry and Molecular Biology)[edit | edit source]
Biotransformation reactions of xenobiotics are catalysed by various enzymes. The most important is oxidation on the P45O. Hundreds of different enzymes have been described in various animal and plant species.
| Major groups of enzymes | Oxidoreductases | Hydrolases | Isomerases | Transferases | Lyases | Ligases |
|---|---|---|---|---|---|---|
| Subgroups | Dehydrogenases Oxidases Reductases Peroxidases Catalases Oxygenases Hydroxylases |
Esterases Glycosidases Peptidases Thiolases Phosphatases Amidases Daminases Ribonucleases |
Racemases Epimerases Isomerases Mutases |
Acetyl- Methyl- Sulfo- Phosphoryl- Transketolases Transaldolases |
Decarboxylases Aldolases Hydratases Dehydratases Synthases Lyases |
Synthetases Carboxylases |
Enzymatic catalysis of Phase I biotransformations[edit | edit source]
- The most important enzymes catalysing phase 1 reactions are hemoproteins showing a characteristic maximum at 450 nm – cytochrome P450 – responsible for the oxidation of about 75% of drugs.
- Cytochrome P450, a mixed oxidase enzyme system, is located on the membranes of the Eendoplasmic reticulum, mitochondria or nucleus; for example, microsomal enzymes on the membranes of microsomes. They exist as isozymes with different amino acid sequences and different substrate specificity.
- freely soluble enzymes – lysoenzymes
- the three main genetic families metabolizing drugs in the liver are: CYP 1, CYP 2, CYP 3
- dozens of P450 isoenzymes: e.g. CYP1A1, CYP1A2, CYP2C9...
- specificity of metabolism - important P450 subtypes and examples of substrates
| P450 | Predominant localisation | Examples of substrates |
|---|---|---|
| CYP1AB | liver | caffeine, theophylline, paracetamol |
| CYP2C9 | liver | ibuprofen, warfarin |
| CYP2C19 | liver | diazepam |
| CYP2D6 | liver, brain, lungs | codeine, tricyclic antidepressants |
| CYP2E1 | liver, lungs, CNS, heart, bone marrow | alcohol |
| CYP3A4 | liver, GI, kidney, lungs, CNS, lymphocytes | erythromycin, nifedipine |
- some xenobiotics cause increased enzymatic activity - induction
- other xenobiotics, on the other hand, suppress enzymatic activity - inhibition. E.g. chronic ethanol abuse leads to induction of CYP2E1 – faster drug metabolism
- P450 and biological variability in animals - the importance of the choice of experimental animals for testing the toxicity of new drugs for human use.
- interindividual variability within a species – genetic polymorphism of enzymes – fast and slow metabolisers – potential occurrence of adverse effects
- variability of the amount of enzyme in tissues during the life of an individual
- Some biotransformation reactions are independent of the P450 system
- oxidative deamination of the NOx series (amphetamine) requires monoamine oxidases (MAOs); found inside cells bound to the surface of mitochondria, in the ER, in nerve endings, in the liver, in the intestinal epithelium
- ethanol is metabolized by the soluble cytoplasmic enzyme alcohol dehydrogenase in addition to the microsomal oxidase CYP2E1
- plasma esterases cause the hydrolysis of NOx, such as procaine or cocaine. They are found in the cytoplasm in many tissues, in the microflora of the GI tract.
| cytochrom P450 dependent processes | Cytochrome P450 independent processes |
|---|---|
| aliphatic hydroxylation | oxidation of primary, secondary, tertiary amines |
| aromatic hydroxylation | oxidative deamination, desulfurisation |
| epoxidation of the double bond | dehydrogenation (alcohol) |
| N. O. S. – dealkylation | reduction of azo, nitro, carbonyl compounds |
| N. S. P. – oxidation | hydrolysis of amides, esters |
| deamination |
Biotransformation of phase II reaction[edit | edit source]
- Phase II reaction: synthetic, conjugation of NOx or metabolites with endogenous substrates – production of very polar metabolites, capable of elimination
- endogenous substrates: glycine, glutathione, glucuronic acid, acetic acid, sulphuric acid
- conjugation enzymes:
- glucuronidation - glucuronosyltransferase (glucuronyltransferase) - an enzyme located in the ER near the cytochrome P450 system
- sulfation - sulfotransferase
- glutathione-S-transferase
- N-acetyltransferase (cytosolic enzyme in various tissues)
- methyltransferases – transfer of methyl from S-adenosylmethionine to a suitable substrate
Oxidation of alcohols[edit | edit source]
Examples:
- methanol: the metabolites undergoes changes - formaldehyde formic acid
- isopropanol: the metabolite is aceton
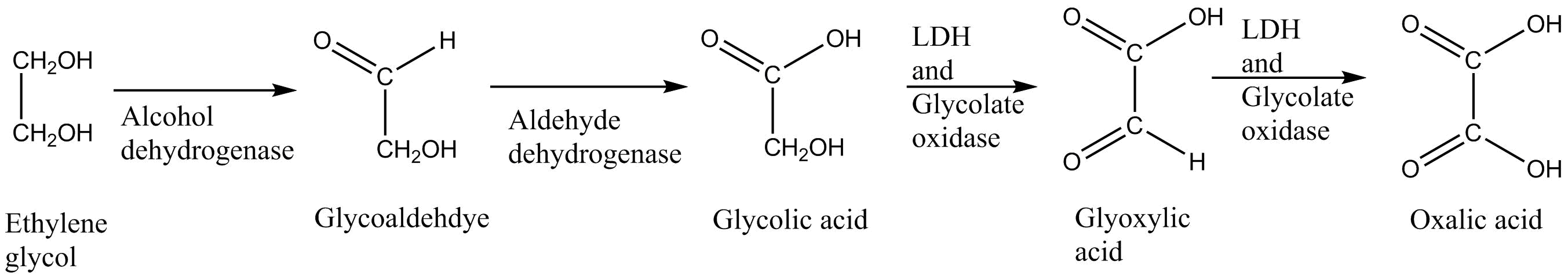
- ethylene glycol: glycoaldehyde glycolic acid glyoxylic acid oxalic acid
Ethyl alcohol[edit | edit source]
- hydrophilic substance, easy absorption (stomach, intestine)
- fat solubility – narcotic effects
- individual manifestations (men, women), abstainers - chronic alcoholics - CNS adaptation, tolerance
- chronic toxicity - damage to the CNS, heart, liver, kidneys - damage to hepatocytes, slowed elimination
- endogenous production by bacterial fermentation of food in the intestine (thousands per thousand)
- postmortem formation of alcohol, putrefactive processes
Kinetics: absorption rate > elimination rate - Elimination 0th order kinetics, linear kinetics
Metabolism[edit | edit source]
- 2-10% of the absorbed dose is excreted by breath and urine, the majority is oxidized in the liver - enzymes ADH (60-70%), MEOS
Oxidation of unsaturated bonds[edit | edit source]
Examples:
- trichloroethylene: the metabolites undergoes changes - trichloroethylene epoxid chloral hydrate trichloroacetic acid
- benzene: benzene epoxid fenol
- styrene: mandolin acid phenylglyoxylic acid
O-Dealkylation[edit | edit source]
Example:
- codeine: the metabolite is morphine
N-Dealkylation[edit | edit source]
Examples:
- codeine: the metabolite is norcodeine
- methamphetamine: the metabolite is amphetamine
- chloroquine: desethylchloroquine didesethylchloroquine
N-Oxidation[edit | edit source]
Example:
- amitriptyline (left): the metabolite is amitriptyline-N-oxide (right)
S-Oxidation[edit | edit source]
Example:
- dosulepin (picture): the metabolite undergoes change - dosulepin-S-oxide dosulepin-S-dioxide
Oxidative desulfurization[edit | edit source]
Example:
- Parathion (left): the metabolite is paraoxon (right)
Oxidative deamination[edit | edit source]
Example:
- Amphetamine (left): the metabolite is phenylacetone (right)
Reduction of aldehydes and ketones[edit | edit source]
Examples:
- Chloralhydrate: the metabolites undergo changes - trichloroacetaldehyde trichlorethanol
- aceton: the metabolite is isopropanol (picture)
Reduction of nitro groups[edit | edit source]
Examples:
- nitrazepam: the metabolite is 7-aminonitrazepam
- nitrobenzene: the metabolite is aniline (picture)
Hydrolysis of esters[edit | edit source]
Examples:
- cocaine: the metabolite can be either ecgonine methyl ester or benzoylecgoninee
- acetylsalicylic acid (aspirin, left): the metabolite is salicylic acid (right)
Hydrolytic Cleavage Cycles[edit | edit source]
Example:
- bromazepam: the metabolite is 2-amino-5-bromobenzophenone
Scheme of biotransformation of chlorpromazine[edit | edit source]
Conjugation of morphine[edit | edit source]
- -OH group of morphine can be conjugated
- e.g. morphine-3-glucosiduronate, morphine-6-glucosiduronate, morphine-3,6-diglucosiduronate, morphine-3-O-sulphate, morphine-6-O-sulphate, morphine-3,6-O-disulphate
Biotransformation of heroin, codeine[edit | edit source]
- heroin can transform into morphine
- codeine can transform into morphine or codeine-6-glucosiduonate
- morphine then can undergo further biotransformation
Biotransformation of cocaine[edit | edit source]
- cocaine is transformed into cocaethylene, norcocaine, benzoylecgonine, methylester ecgonine
- benzoylecgonine and methyl ester ecgonine can then further biotransform into ecgonine
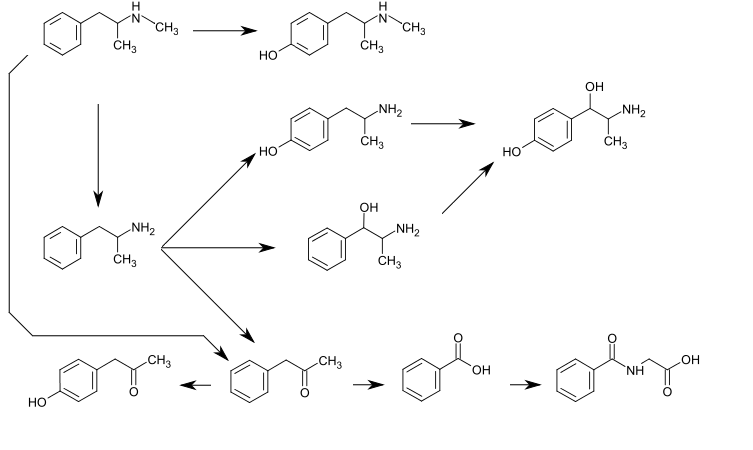
Biotransformation of methamphetamine[edit | edit source]
Biotransformation of benzodiazepines[edit | edit source]
Biotransformation of delta-9-THC[edit | edit source]
- THC (picture) 11-hydroxy-THC 9-carboxy-THC 9-carboxy-THC-mono/diglucosiduronate
Kinetic aspects of biotransformation[edit | edit source]
Knowledge of biotransformation[edit | edit source]
- development and optimization of the toxicological method
- interpretation of toxicological findings
- knowledge of the mechanism of action of xenobiotics for effective treatment of diseases and intoxications, reduction of adverse drug effects
- pharmacokinetics and pharmacodynamics: individual differences (genetic basis, health status, age, gender)
- effects are affected by dose, frequency of doses, method of application, combination of NOx (inhibition or induction of enzymes) etc.
- unexpected manifestations - shock, comatose states
- assessment of toxicological findings, interpretation - a comprehensive point of view with regard to the circumstances of the case
Links[edit | edit source]
Related Articles[edit | edit source]
Source[edit | edit source]
- BALÍKOVÁ, Marie. Osud xenobiotik a biotransformace [online]. [cit. 2012-03-13]. <https://el.lf1.cuni.cz/p88978866/>.
- EYBL, Vladislav. Vybrané kapitoly z obecné farmakologie. Část 1. 1. edition. Karolinum, 2003. 63 pp. ISBN 80-246-0679-8.