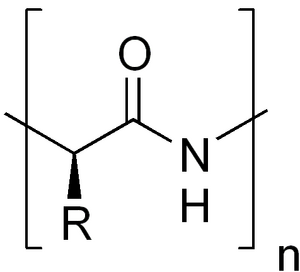
Amino acids are the basic building blocks of proteins. Chemically, they are organic compounds connected to each other by a peptide bond. At least one primary amino group –NH 2 and at the same time at least one carboxyl group –COOH must be present in the amino acid. Chemically, they are substituted derivatives of carboxylic acids.
- 2–100 amino acids (monomers – peptides
- 100 or more amino acids – proteins
More than 700 different AMKs have been demonstrated in nature. That is why we also divide AMK according to their occurrence:
- amino acids found in all living organisms
- bound in proteins (21 proteinogenic AMK), peptides or as free AMK
- amino acids found only in some organisms
- bound in peptides or as free AMK
- they are not components of proteins
Proteinogenic amino acids, or coded ones, occur in proteins as L-alpha-amino acids (the exception is glycine). This is due to the chemical arrangement that is necessary for biogenic function. Specific types of amino acids, their sequence and spatial structure then give proteins their biological properties.
- amino group (-NH2, free, substituted)
- carboxyl group (-COOH)
- other functional groups
- hydroxyl -OH
- sulfhydryl (mercapto group) -SH
- sulphide -S-R
- guanidyl
- phenyl etc.
- according to the structure of the side chain and functional groups
- according to side chain polarity
- according to importance in human nutrition
- essential = the human organism is unable to create them endogenously
- conditionally essential = essential in the absence of precursors or immaturity of enzymatic systems
- completely non-essential
| Extended classification
|
- 19 α-amino acid with a primary amino group (-NH2)
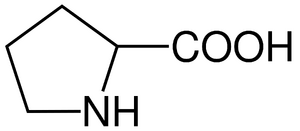
- 1 α-amino acid with a secondary amino group (-NH-)
n=0, pyrrolidin
- 18 amino acids = chiral compounds of the L series
- trivial names, systematic names, symbols (three-letter, one-letter)
Classification of essential amino acids[edit | edit source]
By side chain structure and functional groups
- aliphatic with an unsubstituted chain
- aliphatic hydroxyamino acids
- aliphatic sulfur
- with a carboxyl group in the side chain (monoaminodicarboxylic, acidic)
- their monoamides (with a carboxamide group in the side chain)
- with basic groups in the side chain
- with an aromatic (heterocyclic) side chain
According to the polarity of the side chain and its ionic form (in a neutral environment)
- non-polar, hydrophobic
- Val, Leu, Ile, Phe, Tyr, Met, Pro;
- sometimes Gly, Ala, Trp (amphiphilic)
- polar, hydrophilic
- Ser, Thr, Cys, Asp, Glu, Asn, Gln, Lys, Arg, His
- Hydrophilic (according to the ionic form of the side chain in a neutral environment)
- neutral (has no electrical charge): most
- acidic (negative charge): Asp, Glu
- basic (positive charge): Lys, Arg, His
|
|
| Representatives
|
Derivatives of basic proteinogenic amino acids[edit | edit source]
- the emergence of specific modifications
- L-cystin (CySSCy)
- 4-hydroxy-L-prolin (Hyp)
- 5-hydroxy-L-lysin (Hyl)
- 3-methyl-L-histidin
- O-phospho-L-serine
Other non-protein amino acids[edit | edit source]
N-substituted α-amino acids[edit | edit source]
- N-methylglycin (sarkosin) , N,N-dimethylglycin , N,N,N-trimethylglycin
- L-carnitine (3-hydroxy-4-trimethylaminobutyrate, vitamin Bt)
- ß-alanine (3-aminopropionová kyselina) , γ-aminobutyric (4-aminobutyric) acid (GABA)
- S-alk(en)yl-L-cysteiny , S-alk(en)yl-L-cysteinsulfoxidy
Basic amino acids and related compounds[edit | edit source]
- L-ornithin (n = 2)
- L-citrulline (n = 2, karbamoylderivát ornithinu)
- creatine-phosphate
foods deficient in certain amino acids
- Lys − cereals (plant proteins in general)
- Met − milk, meat
- Thr − wheat, rye
- Trp − casein, corn, rice
|
|

S-alk(en)yl-L-cysteinesulfoxid
| Physico-chemical properties
|
Physico-chemical properties[edit | edit source]
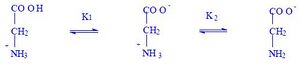
Acid-base properties (Gly)[edit | edit source]
| ion I1 (kation) |
ion I2 (amfion) |
ion I3 (anion)
|
| free charge +1 |
free charge 0 |
free charge -1
|
| pH < 2 |
pH ≈ 6 |
pH > 10
|
Dependence of the ionic forms of Gly on pH
cation (I1) → amphion (I2) → anion (I3)
- Gly = exception
- majority = chiral atom Cα... 2 optical isomers (enantiomers)
- some 2 chiral centers... Ile, Thr, Hyp, CySSCy
L- and D-amino acids, L-amino acids = (S)-stereoisomers,výjimka: L-cysteine = (R)-stereoisomer
D-amino acids = (R)-stereoisomers
Content
Diastereoisomers of amino acids
- sweet - Gly, Ala, Thr, Pro
- acidic - Asp, Glu
- bitter - Leu, Ile, Phe, Tyr, Trp
- indifferent - others
Unique properties = umami taste
|
|

sodium hydrogen glutamate
- VELÍŠEK, Jan – HAJŠLOVÁ, Jana. Chemie potravin 2. 3. edition. 2009. ISBN 978-80-86659-17-6.
- SVAČINA, Štěpán – ET AL.,. Klinická dietologie. 1. edition. 2008. ISBN 978-80-247-2256-6.