X-rays
X-rays were discovered in 1895, essentially by accident, by Wilhelm Conrad Röntgen.
X-rays are electromagnetic ionizing radiation with a wavelength of 10 nm–1 pm (10−8–10−12 m). Due to quantum duality, they can also be viewed as photons with an energy of 5–200 keV sufficient to knock an electron out of the atomic envelope (ionization).
Sources of X-ray radiation[edit | edit source]
- Natural sources of X-rays are radiation from stars (e.g. the sun), but also other cosmic sources.
- Artificial sources of X-rays are, for example, X-ray tubes.
Types of X-rays[edit | edit source]
The origin of X-rays is in the electron shell. Depending on the wavelength we can distinguish 2 types of X-rays, soft (with a larger wavelength λ= 10−8−10−10m) and hard (λ= 10−10−10−12m). The X-ray sources used produce two types of radiation (according to the different energy distribution in the spectrum):
Braking X-rays[edit | edit source]
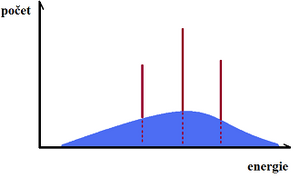
Electrons flying fast (at 100 kV their velocity is about 165,000 m/s) from the cathode to the anode are thrown into a strong electrostatic field when they strike the anode, where their path is curved and braked. The kinetic energy lost by the electron is radiated in the form of a photon of X-ray radiation. In this process, photons of different wavelengths are emitted. The closer the electron gets to the nucleus and the greater its energy, the greater the energy of the resulting X-ray quantum. The energy of the braking X-ray depends on the proton number of the anode and the velocity of the electrons (i.e. the magnitude of the voltage between the electrodes of the X-ray tube). This radiation is characterised by a broad continuous energy spectrum because the velocity of the electrons emitted by the cathode is not uniform. Brake X-rays produce a continuous spectrum. However, electrons can be accelerated in other ways than simply by exposure to very high voltages - in particle accelerators such as linear accelerators, betatrons or microtrons, much higher energies are achieved than in X-rays, and the resulting X-rays are much harder.
Brake radiation is used in medical diagnostics and radiotherapy, or in industry in defectoscopy.
Characteristic X-rays[edit | edit source]
The characteristic X-rays vary according to the material of the anode. Electrons striking the anode (usually tungsten) transfer their energy to electrons in the atoms of the anode, these electrons are excited (knocked to a higher energy level) or completely ionized (knocked out of the envelope). If the electron has only been excited, it subsequently returns to its original ground state; if it has been "knocked out", then its place is filled by an electron from one of the more energy-rich levels further away from the nucleus. In both variations of electron descent, a significant amount of energy is released in the form of X-rays. The energy of the photon of radiation is equal to the energy difference between the electron levels between which the electron was moved. The energy difference between the levels is always the same, so only certain wavelengths of X-ray radiation are produced - hence the name characteristic radiation, because the energy difference that determines the wavelength of the radiation depends on the material of which the anode is made. Thus, we obtain X-ray radiation characteristic of a particular element (the anode material); its energy is higher the higher the proton number of the element forming the anode. The resulting radiation forms a so-called discrete - line spectrum.
Characteristic X-rays are used in analytical chemistry.
The resulting radiation of a real X-ray source is the sum of the stop and characteristic radiation.
X-ray properties[edit | edit source]
(applied in diagnostics)
- Ability to penetrate matter - this ability depends on the properties of the absorbing matter and the energy of the radiation (the energy is greater the shorter the wavelength);
- Differential absorption - the ability of different substances to absorb X-rays depends, for example, on the proton number of the elements of the absorbing tissue, the thickness of the layer (bone - Ca, P → large absorption);
- Photochemical effects - causes blackening of photographic plate or film (depends on intensity of radiation);
- Luminescence effects - production of visible radiation when incident on certain materials;
- Direct propagation from source - propagates into space in all directions and intensity decreases with the square of the distance;
- Radiation scattering - negative property for diagnostics, reduces contrast; when a photon interacts with an electron, the beam is deflected and energy is reduced (wavelength increases);
- Ionization effects - negative property, may have harmful biological effects.
X-ray tube[edit | edit source]
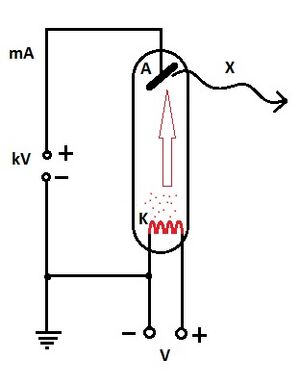
It is a vacuum tube containing 2 electrodes: cathode and anode. Most often both are made of tungsten, for mammographic examinations a molybdenum anode is used (softer X-rays). To reduce radiation dose and increase sharpness during imaging it is shielded (most often with lead). A very high voltage is applied to the electrodes, which leads to acceleration of the electrons.
Cathode[edit | edit source]
The cathode has the shape of a spiral. When the cathode heats up, electrons are emitted and an electron cloud is formed. The density of the cloud is determined by the incandescent current of the cathode. When a high DC voltage is applied, negatively charged electrons start to fly out of the cloud towards the anode (+ simultaneous acceleration by a strong electric field). When decelerating at the anode, only 1% of the electrons' motion energy is radiated.
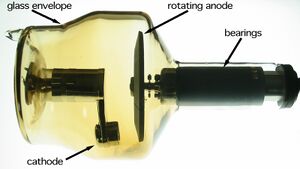
Anode[edit | edit source]
The anode can be fixed or rotating. When electrons hit the anode, it heats up and therefore cooling is necessary. For low power X-ray tubes, air cooling is sufficient. A high power X-ray tube has a cavity inside for the cooling effect.
Principle[edit | edit source]
In an X-ray tube, the voltage between the cathode and anode (anode voltage) and the intensity of the cathode current (cathode current) can be independently controlled.
The intensity of the radiation depends on the cathode current. The higher the cathode current, the higher the radiation intensity.
The hardness, penetration, absorption and wavelength of the radiation depend on the anode voltage. As the anode voltage increases, the hardness and penetration of the radiation will increase and conversely the absorption and wavelength will decrease. The greater the potential between the cathode and anode, the greater the acceleration of the electrons and the shorter the wavelength of the X-ray radiation produced.
Radiation parameters[edit | edit source]
- photon energy - is directly proportional to the voltage between cathode and anode;
- intensity of radiation - related to the cathode's heating current;
- applied voltages - 20-80 kV, for conventional imaging about 70 kV, for mammography 20-30 kV;
- radiation energy - higher energy results in lower quality of imaging, lower energy results in higher quality of imaging (contrast), but at the same time results in higher radiation load on the patient;
Radiation detection[edit | edit source]
- film - in scanning, it is a photochemical process;
- fluorescence screen - in scanscopy;
- digitally - in CT, stimulation of the phosphor layer on the recording medium, subsequent luminescence detected digitally;
- CCD - direct digitization, in visiography;
- fixed large-area detectors for digitalisation, in direct radiography (DR).
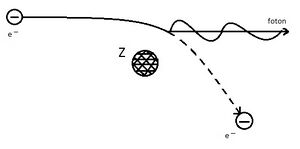
Compton effect[edit | edit source]
It is the elastic scattering of X-ray photons on free electrons, where the wavelength of the scattered radiation is larger than the wavelength of the incident radiation. The frequency of the scattered radiation is less than the frequency of the incident radiation.
The Compton shift is the difference between the wavelength of the incident radiation and the wavelength of the scattered radiation. The Compton effect demonstrates the particle nature of X-ray radiation. However, X-rays also have wave properties, as evidenced by their ability to polarise and diffract.
Use in medicine[edit | edit source]
X-rays can pass through human tissues and, as a result of their passage, produce shadow-like images of structures in the body (e.g. bones, certain organs and, last but not least, various pathological conditions). An X-ray image is a still image; in simple terms, it is an X-ray photograph. One of the best known devices using X-rays in medicine is without doubt CT (Computed Tomography). The characteristic feature of X-rays is that they have much higher energy than visible light, and this is partly absorbed as they pass through the human body. The absorbed energy of X-rays can also have various biological effects within the tissue, and the amount is called the radiation dose. Very high doses of radiation are used in radiation oncology or therapy to stop the multiplication of cancer cells. However, the radiation dose during imaging is very small. The smallest possible radiation dose is used to produce the necessary image quality in diagnostic imaging.
Other uses:
- X-ray diagnostics - calcium-dominated bones absorb X-rays more than muscles and tissues (mostly water). Bones appear lighter on the X-ray image (negative);
- X-ray structural analysis;
- X-ray defectoscopy;
- In archaeology;
- X-ray astronomy;
- Scanography;
- Skiascopy;
- Angiography;
Links[edit | edit source]
Related articles[edit | edit source]
References[edit | edit source]
SVOBODA, Emanuel. High school physics overview. 4. edition. Prague : Prometheus, 2010. ISBN 9788071963073.
NAVRÁTIL, Leoš – ROSINA, Jozef. Medical biophysics. 1. edition. Prague : Grada, 2005. pp. 524. ISBN 80-247-1152-4.
TARÁBEK, Pavol. Odmaturuj z fyziky. 2. edition. Brno : Didaktis, 2004. ISBN 8073580586.