Urine / chemical examination
Protein, glucose, hemoglobin, ketone bodies and bile pigments are routinely detected in urine. These components are usually found in the urine of healthy people, but in such small amounts that we do not prove them by routine tests. In various pathological conditions, their concentration in urine increases.
Tube reactions[edit | edit source]
__ Chemické vyšetření moči mokrou cestou
Test (diagnostic) strips[edit | edit source]
Detection of pathological components of urine directly at the patient's bedside or in the line of first contact with the patient enables examination using test strips.
The test strips consist of a plastic carrier on which one or more indication zones are mounted. These are manufactured in such a way that a liquid analytical reagent is sucked into a suitable material (eg special filter paper) and gently dried.
Diagnostic strips are available as monofunctional, multifunctional or special examination strips.
Monofunctional strips contain basic indication zones for semi-quantitative determination of a substance in urine. Polyfunctional strips are formed by several indication zones, enabling examination of several biochemical parameters at once. They are intended for cases where it is necessary to obtain as much information as possible about the patient's health condition, eg at various screening events. In addition to monofunctional and polyfunctional strips, there are special test strips that include combinations of two or more indication zones that are selected for a particular disease, e.g., diabetes mellitus screening strips contain a zone for glucose, ketone bodies, protein, and pH.
The following urine parameters can be determined using test strips:
- protein;
- glucose;
- ketone bodies;
- bilirubin;
- urobilinogen;
- hemoglobin, erythrocytes;
- ascorbic acid;
- leukocytes;
- nitrite;
- pH;
- density.
Principles of determining individual parameters[edit | edit source]
Protein[edit | edit source]
__
The principle of determination of proteins in urine using diagnostic strips is based on the so-called protein error of an acid-base indicator , eg tetrabromophenol blue , tetrabromophenolphthalein ethyl ester or 3´, 3´´, 5´, 5´´-tetrachlorophenol-3,4,5,6- tetrabromosulfophthalein. Like any acid-base indicator, these substances change color at a certain pH (they behave like weak acids, while the protonated form has a different color than the dissociated form): at pH below 3.5 they are yellow, at higher pH they are green to blue. In addition to the indicator, there is a buffer in the reaction zone of the test strip, which maintains the pH in the range of 3.0 to 3.5, so the indicator is yellow. If there are proteins in the sample, they bind to the indicator with their amino groups. However, this changes its properties - the transition area shifts towards a more acidic pH. This means that at the stated constant pH between 3.0 and 3.5, the protein-bound indicator will be green, as if it were in a more alkaline environment (hence the protein error of the indicator ).). The color intensity depends on the protein concentration, varies from green to blue and is evaluated visually or instrumentally.
In highly alkaline urine (pH above 8) or if the urine is very concentrated, the test may give false positive results (buffer will be depleted in the reaction zone). In these cases, acidify the urine with a few drops of dilute acetic acid to pH 5-6 and repeat the test. False positives can also be caused by high concentrations of some substances with amino groups (contamination of the sampling vessel with some disinfectants), which bind to the indicators similarly to proteins.
The disadvantage of the test strips is their different sensitivity to individual proteins. The strips react very well with albumin and indicate its presence in urine from 0.1 to 0.5 g / l. They are significantly less sensitive to globulins, glycoproteins and Bence-Jones protein . These diagnostic strips do not show an increase in albuminuria to values up to about 200 mg / l, resp. daily albumin losses in the range of 30 to 300 mg / 24 hours, which accompanies especially the earlier phases of some nephropathy. Immunochemical methods can be used to screen for increased albuminuria, such as special diagnostic strips based on immunochromatographic principles or immunoturbidimetry.
Hemoglobin[edit | edit source]
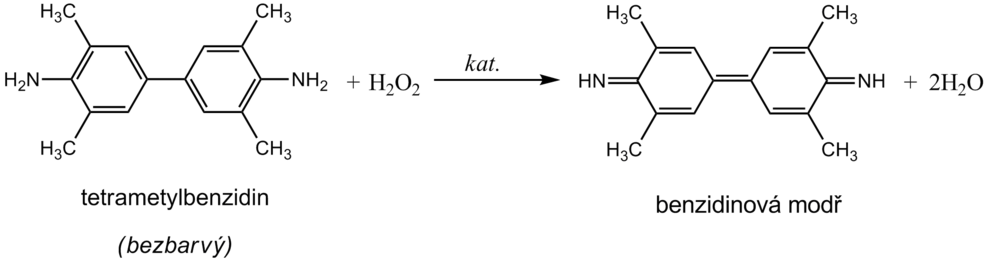
__ Hemoglobin catalyzes, like peroxidase , the oxidation (dehydrogenation) of some substrates (eg benzidine derivatives ) by hydrogen peroxide:
However, it is not an enzyme activity (catalysis is conditioned by heme iron) and therefore it is not lost even after heat denaturation. We are talking about pseudoperoxidase activity , which is used for sensitive but non-specific evidence of hemoglobin or trace amounts of blood. It is preferable to use a chromogenic substrate to monitor the reaction, i.e., a substance that provides a markedly colored product by dehydrogenation (often benzidine or its non-carcinogenic derivatives, aminophenazone, etc.).
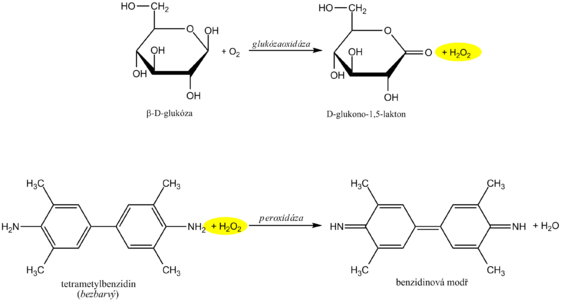
The reagent zone of the diagnostic strips contains a chromogen (eg tetramethylbenzidine ) with stabilized hydrogen peroxide (eg cumene hydroperoxide ). In the presence of free hemoglobin ( hemoglobinuria ), the indication zone turns uniformly blue. If erythrocytes ( erythrocyturia ) are present in the urine , intensely green-blue dots to spots form.
Hemoglobinuria can be encountered in intravascular hemolysis . Damage to the glomerular membrane ( glomerular hematuria ) and bleeding from any part of the urinary tract lead to more frequent erythrocyturia . It is often found in urinary tract infections , urolithiasis and urogenital tract tumors .
In addition to hemoglobin , myoglobin also provides a pseudoperoxidase response , which can be excreted in the urine during skeletal muscle breakdown ( rhabdomyolysis , crush syndrome ). The positivity of the test may also be due to peroxidases of leukocytes or certain bacteria, yeasts or fungi, which may occur in the urine, especially in urinary tract infections . To rule out the possibility of a false positive reaction due to cellular peroxidases, the reaction must be performed with boiled urine.
Contamination of the sampling vessel with strong oxidizing agents also causes a false positive reaction. On the other hand, the presence of strong reducing substances (eg ascorbic acid ) can slow down or even stop the pseudoperoxidase reaction and thus cause false negative results.
Glucose[edit | edit source]
__
Diagnostic strips for the detection of glucose in urine are based on the principle of enzymatic reactions with glucose oxidase and peroxidase (same principle as the determination of glycemia ). D -glucose is oxidized by oxygen using glucose oxidase to form D -glucono-1,5-lactone and hydrogen peroxide. In a subsequent peroxidase reaction, hydrogen peroxide oxidizes tetramethylbenzidine or another chromogen to a colored product. The light yellow color of the reaction surface changes to blue-green when positive. The test is specific for D -glucose, other sugars do not give a positive reaction.
High concentrations of reducing agents such as ascorbic acid slow down the development of color and can lead to falsely lower results. In these cases, it is recommended to repeat the analysis at least 10 hours after vitamin C withdrawal. Conversely, false positive results may be due to the presence of peroxidase substrates or oxidizing agents in the sampling vessel (eg H 2 O 2 , Persteril ®, chloramine B ). Urine glucose determination should be performed rapidly to avoid bacterial contamination or urine should be stored at 4 ° C.
Interference with ascorbic acid is a common source of false negatives. Urine test strips from some manufacturers are therefore modified so that the reaction zone is at least somewhat resistant to ascorbic acid. Some diagnostic strips also have an ascorbate detection zone to alert you to false negatives.
Glucosuria is most often accompanied by an increase in glycaemia above the so-called renal glucose threshold (around 10 mmol / l). Glucose, which normally filters through the glomerular membrane, is in such a high concentration in primitive urine that it is not enough to be resorbed in the tubules and reaches the final urine. Glucosuria with normal glycemia indicates a disorder of tubular transport mechanisms - we are talking about renal glucosuria .
Glucosuria is most often accompanied by an increase in glycaemia above the so-called renal glucose threshold (around 10 mmol / l). Glucose, which normally filters through the glomerular membrane, is in such a high concentration in primitive urine that it is not enough to be resorbed in the tubules and reaches the final urine. Glucosuria with normal glycemia indicates a disorder of tubular transport mechanisms - we are talking about renal glucosuria.
Ketones[edit | edit source]
__ The detection of ketone bodies is based on the reactions of acetoacetic acid and acetone with sodium nitroprusside in an alkaline medium, which forms a red-violet-colored complex . This principle is used by Legal's and Lestradet's exams , as well as diagnostic strips. Β-hydroxybutyric acid (ie the most abundant ketone substance) does not provide a reaction and therefore a negative result does not completely rule out ketoacidosis.
Compounds with free sulfhydryl groups (eg the antihypertensive captopril or uroprotectant used in some mesna chemotherapeutic regimens ) provide false positivity for urinary ketone bodies . Quite often, bacterial products in urinary tract infections also provide a similar response.
False negatives , apart from the already mentioned insensitivity of the β-hydroxybutyric acid tests, are not significant.
Viz též Ketolátky v moči.
Bilirubin[edit | edit source]
__ Detection of bilirubin in urine by diagnostic strips is based on the azocoupling reaction provided by conjugated bilirubin with a stable diazonium salt (eg 2,6-dichlorobenzenediazonium tetrafluoroborate ). A pink to pinkish-red dye is formed. When high concentrations of urobilinogen occur, the color changes to orange. In this case, it is recommended to evaluate the coloration up to 2 minutes after the indication zone has wetted. Lower to false negative results may be due to high concentrations of ascorbic acid . Urine specimens should be protected from direct sunlight, which causes oxidation of bilirubin, followed by a falsely inferior to negative finding.
Only conjugated bilirubin is examined in the urine , as unconjugated bilirubin cannot be excreted in it.
Urobilinogen[edit | edit source]
__ As with bilirubin, the principle of azocoupling reaction with a stable diazonium salt (eg 4-methoxybenzenediazonium tetrafluoroborate) is used to determine urobilinogen in urine . The indication zone turns pink to red in the presence of urobilinogen. The faint pink color still corresponds to the physiological excretion of urobilinogen. In the presence of bilirubin , the color is yellow, which turns green to blue after 1 minute.
Some heterocyclic nitrogenous substances produced by bacteria in urinary tract infections can cause false positives . High concentrations of ascorbate can cause false negatives .
Leukocytes[edit | edit source]
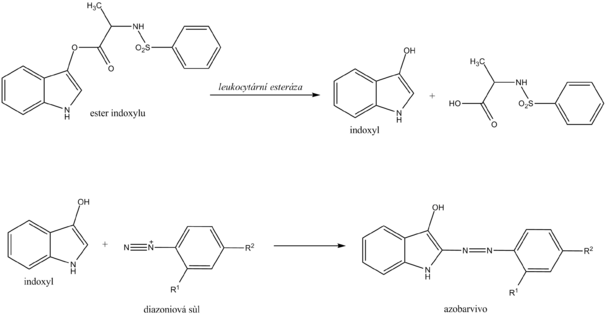
__ Chemical determination of leukocytes with a diagnostic strip is based on the detection of esterases that are abundant in granulocytes . Granulocyte esterases catalyze the hydrolysis of indoxyl ester to free indoxyl. The indoxyl then reacts with the stable diazonium salt to form the appropriate azo dye. In the case of a negative reaction, the zone turns cream yellow; in the case of a positive reaction, it changes to a pink to purple hue.
Chemical examination of leukocytes does not replace microscopic examination . On the other hand, it is possible to detect lysed leukocytes (eg in hypotonic urine ) in this way, which is not possible with microscopic examination.
Leukocyturia is a symptom of inflammation of the kidneys or urinary tract. The cause of most positive findings is a bacterial infection of the urinary tract . In case of a positive leukocyte finding, it is recommended to supplement the examination of proteinuria , hematuria , nitrituria , examination of urinary sediment and further microbiological examination .
Nitrite[edit | edit source]
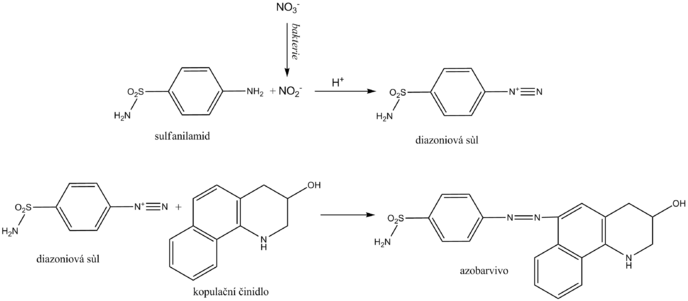
__ Nitrite is determined in the urine as an indirect sign of bacteriuria . Normal urine does not contain them in measurable concentrations. Some primarily gram -negative bacteria , such as Escherichia coli , Proteus , Klebsiella , staphylococci and others, have the ability to reduce the nitrates present in the urine to nitrite. Diagnostic strips for indirect detection of bacteriuria use nitrite in the so-called Griess reaction . Its essence is the diazotization of sulfanilamide with nitrite in the sample to form the diazonium salt. This is followed by azocoupling of the resulting salt with a coupling agent, developing a pink to purple color.
The nitrite urine test should be performed in the first morning urine, as in this case a sufficiently long time is guaranteed for the bacterial reduction of nitrates to nitrite in the bladder. Another recommendation is to consume enough vegetables (contains nitrates) the day before the examination. A positive urinary nitrite test confirms bacteriuria, while a negative one does not excrete it.
Indirect evidence of bacteriuria is indicative and does not replace microbiological examination.
Ascorbic acid[edit | edit source]
__ Ascorbic acid appears in the urine with high food intake. As a strong reducing agent, it can affect the determination of some analytes in urine, especially those that use hydrogen peroxide in the reactions. It is directly reduced with ascorbic acid. It also rapidly degrades diazonium salts used for azocoupling reactions.
The principle of ascorbic acid detection uses phosphomolybdic acid, which is reduced to molybdic blue by ascorbic acid. The reaction is not only specific for ascorbic acid, other substances with strong reducing effects react similarly.
pH[edit | edit source]
__
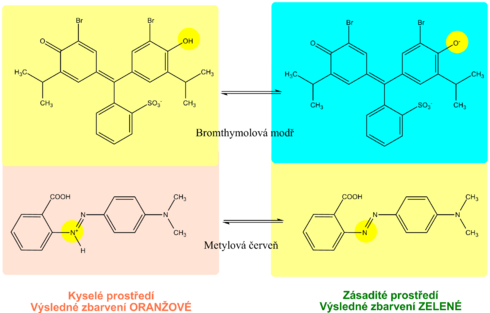
The pH indication zone contains a mixture of selected acid-base indicators. Most use two acid-base indicators - methyl red and bromothymol blue, or phenolphthalein . This ensures that the pH is read in the range 5-9 by changing the color from orange (acidic pH) to green to blue (alkaline pH). The reading is possible with an accuracy of 0.5.
Density[edit | edit source]
__ By relative urine density we mean the ratio of urine density to water density. The density of water is practically equal to 1 kg / l, so the difference between the density of water (in kg / l) and the relative density of urine is negligible. The density in the SI system is kg · m -3 . The density of the sample relative to the density of water is a relative quantity and is therefore given by a dimensionless number.
Determination of urine density[edit | edit source]
Urine density is estimated indirectly from cation concentrations using diagnostic strips. The indicator zone of the strip contains a suitable polyelectrolyte as an ion exchanger and the acid-base indicator bromothymol blue. The principle of diagnostic strips is based on the exchange of cations from urine, especially Na + , K + , NH 4 + , for H + polyelectrolyte ions in the indication zone. The released H + acidifies the weakly buffered acid-base indicator, which is in alkaline form. Acidification is accompanied by a change in the color of bromothymol blue. The disadvantage is that the examination with diagnostic strips does not take into account non-electrolyte substances such as glucose , proteins, urea ,creatinine and some others.
Procedure for examination with diagnostic strips[edit | edit source]
We will only remove as many strips from the tube as we need immediately. Close the tube with the remaining strips immediately to protect the unused strips from moisture. We do not touch the strip indication zone with our hands. We store the strips only in the original packaging and well closed with a bag of desiccant, in the dark, in a dry place at a temperature of +2 to +30 ° C.
We immerse the strip briefly for 1-2 s in the examined urine so that all zones are wetted. Then remove the strip and remove excess urine by wiping the edge of the strip against the edge of the container. We then place it in a horizontal position to prevent mixing of reagents from the individual reagent pads. After the prescribed reaction time has elapsed, usually 60 s and for leukocytes 120 s, we evaluate.
Evaluation of the color of the reaction zones of the diagnostic strips is performed:
- subjectively comparing the resulting coloration to the color scale on the tube label in which the strips are stored;
- objectively using reflection photometers that measure the intensity of light of the appropriate wavelength reflected from the reaction field.
Overview of urine tests using diagnostic strips[edit | edit source]
| Analyze | Principle | False positive results | False negative results |
|---|---|---|---|
| Protein | Protein error of acid-base indicator | Alkaline pH, contamination of the sampling vessel with disinfectants based on quaternary ammonium salts, menstruation (not examined 3 days before and after), hemoglobinuria, myoglobinuria | Globulins and immunoglobulin light chains are difficult to detect |
| Hemoglobin | Oxidation of chromogen by hydrogen peroxide due to pseudoperoxidase activity of hemoglobin | Microbial peroxidases, contamination of containers with oxidative cleaning agents | High nitrite concentration, vitamin C |
| Glucose | Glucose oxidase reaction coupled with peroxidase reaction | Contamination of containers with oxidative cleaning agents | Vitamin C, other reducing substances (gentisic acid, DOPA), urinary tract infection |
| Ketones | Reaction of acetoacetic acid and acetone with nitroprusside in an alkaline medium | Substances with free sulphhydryl groups (eg captopril). Some phenolphthalein and sulfophthalein-based substances (laxatives and diagnostics) provide similar coloration in alkaline environments. Phenylpyruvic acid. | β-hydroxybutyrate does not react |
| Bilirubin | Azocoupling reactions | Substances that have a similar color in an acidic environment, nitrogenous metabolites of bacteria in some urinary tract infections | High nitrite content, light exposure, ascorbic acid |
| Urobilinogen | Azocoupling reactions | Substances that have a similar color in an acidic environment, nitrogenous metabolites of bacteria in some urinary tract infections | Formaldehyde, light exposure, old urine, ascorbic acid |
| Nitrite | Griess reaction - diazotization of sulfanilamide with nitrite and subsequent azocoupling | Bacterial contamination | Dietary nitrate deficiency, Gram-positive bacteria, major diuresis, vitamin C |
| Leukocytes | Esterase activity of granulocytes and macrophages | Formaldehyde, alkaline pH, high urine density | Vitamin C, some drugs |
| pH | A mixture of acid-base indicators | Formaldehyde (seemingly lower pH), old urine (alkaline pH) | |
| Density | Ion exchange of urine cations for H +, determination of pH by acid-base indicator | Alkaline pH pushes the results to lower values |
Links[edit | edit source]
Reference[edit | edit source]
1-HOHENBERGER, E.F. and H KIMLING. Compendium urinanalysis. Urinalysis with test strip [online] . 1st edition. Mannheim : Roche Diagnostics, 2008. 105 pp. Also available from <http://www.diavant.info/diavant/servlet/MDBOutput?fileId=1392>.
2-PLIVA-Lachema Diagnostics. PHAN® diagnostic urine test strips [online]. Last revision 2009-12-04, [cit. 2010-03-21]. <https://www.erbalachema.com/cz/>.
External links[edit | edit source]
- DASTYCH, Milan and Jaroslav WINKLER. Automated urinalysis [online]. Portal of the Faculty of Medicine of Masaryk University, ©2005. Last revision 2011-10-27, [cit. 2011-11-25]. <http://portal.med.muni.cz/clanek-10-automaticka-analyza-moci.html>.
References[edit | edit source]
- SCHNEIDERKA, Petr, et al. Chapters in clinical biochemistry. 2nd edition. Prague: Karolinum, 2004. 365 pp. ISBN 80-246-0678-X.

![{\displaystyle \mathrm {H} _{2}\mathrm {O} _{2}+\mathrm {H} _{2}\mathrm {A} \ {\xrightarrow[{\mathrm {nebo\ hemoglobin\ a\ jin{\acute {e}}\ l{\acute {a}}tky} }]{\mathrm {peroxid{\acute {a}}zy} }}\ 2\ \mathrm {H} _{2}\mathrm {O} +\mathrm {A} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/23377fd4d265df004d50c7657f43bcbbe4cdc8ff)