The interactions between ionizing radiations and atomic electron cloud
- Previous chapter: 5.2.3 Types of radioactive decay
During the passage through an absorbent matter, the ionizing radiation loses its energy. The way this energy is lost depends on the type of ionizing radiation and on the physical properties of the absorbent matter. The processes taking part in these energy losses during the interactions of ionizing radiation with the electron cloud are as follows:
- excitation
- ionization
- scattering
- bremsstrahlung
Excitation is the process during which an atom of the absorbent matter absorbs the energy of ionizing radiation, leaves the ground state, and enters the excited state. The atom remains in an excited state for a short period of time (10-5 – 10-8 s). During the following the return to the ground state (de-excitation), the difference between energy levels is released in a form of an electromagnetic radiation quantum.
An electron can be supplied with higher energy than the required binding energy during the interaction with the ionizing radiation. Then, the surplus energy is transformed into the kinetic energy of the liberated electron. Ionization is the process of liberating an electron from the electron cloud of an atom exposed to ionizing radiation. The original neutral atom then changes into a positively charged ion. The energy needed to create one ion pair in the air is about 34 eV. Due to the interaction with electron cloud, the ionizing particle gradually lowers its kinetic energy down to the values corresponding with thermal motion. Ionizing particles with certain kinetic energy lose this energy over their trajectory due to the interaction with the electron cloud of the absorbent element. This trajectory is called the range of the particle.
Primary ionization is the number of ion pairs created by the ionizing particle. An ion pair is a positively charged ion plus an electron. Some of the electrons liberated by the primary ionisation can gain such high levels of energy that they themselves can ionize their surrounding environment. The ionization caused by liberated electrons, is called secondary ionisation. Total ionization is the sum of primary and secondary ionization.
Specific linear ionization is the decrease in energy of the charged particle caused by ionisation over the length of its decay track. With the levels of energy of the emitted particles being very high, the measurable decrease in ionization is considerably small. Maximum decrease is observed in particles with lower energy. This means that emitted particles with lower kinetic energy reaches higher number of created ion pairs per unit track length than the particles with higher energy. As it is evident, specific linear ionization is a function of the size of the charge of the particle, the particle’s mass, and its energy.
The track of the ionizing particle in the absorbent medium does not have to be linear, but can be curved in various angles. The curvature of the track is called particle scattering.
Directly ionizing alpha radiation and its interaction with the electron cloud[edit | edit source]
The large mass of the α-particles and their rather strong electric charge (two positive elementary charges) causes the ionizing losses of energy during the passage through an absorbent matter to be quite significant. With particles of the same mass, the ionizing abilities increase with the charge of the particle. This is why α-particles cause more ionization than protons or deuterons. The α-particles show a highly specific linear ionization; over the track of 1cm there are tens of thousands of ions produced. This means that their track is linear. The energy needed to create one ion pair in the air (approximately 34 eV) is practically independent on the energy of the particles. Because the ionizing losses are quite large, the range of α-particles is very short in the air, given energies about 10 MeV. Alpha particles reaches only ranges of about 10 cm. Approximately half of the energy losses of the ionizing α-particle is used for the ionization itself. The other half is used to excite the atoms found in the surrounding environment. Another type of α-particles interaction, called elastic and inelastic collision, is practically insignificant.
When α-radiation comes into contact with human skin it is immediately absorbed in the upper layers of epidermis. This is because the range of α-particles in tissue is only a few micrometres. A-radiation is not dangerous when applied externally, with the possible exception of the eye. However in the case of internal application, the energy of α-particles is absorbed in a very small volume of tissue and therefore has very harmful biological effects. Similar to α-radiation, other charged heavy particles such as protons, deuterons, etc., display a significant specific linear ionisation.
Directly ionizing β-radiation and its interaction with the electron cloud[edit | edit source]
The largest loss of the electrons energy during its passage through an absorbent medium is due to the process of ionization and excitation. Compared to α-radiation, electrons possess smaller mass and charge. Therefore their specific linear ionization is a lot smaller than the one of α-particles. This also means that their range is greater. Another phenomenon gaining larger significance in the case of electrons is the elastic scattering and the origin of so called bremsstrahlung. Scattering is the real distance travelled by an electron, which in an absorbent medium is about 4 times longer than its range. Bremsstrahlung means “braking radiation“ or “deceleration radiation“, and is considered for the wavelength of X-radiation.
Bremsstrahlung is an electromagnetic wave motion produced by the deceleration of a moving electron in the electrostatic field of a nucleus. The deceleration happens due to Coulomb interactions. When the electron decelerates there is an electromagnetic wave motion produced in the direction of its original movement. The intensity of this wave motion is directly proportional to the proton number of the absorbent medium and to the energy of the electrons. The ratio between the energy losses due to bremsstrahlung and ionization depends on the energy of the emitted electrons. For lower energies, the energy loss caused by bremsstrahlung is quite small. The losses only become significant when the emitted electrons possess higher energy levels. Bremsstrahlung has a continuous spectrum. This is because the size of the interaction between the particle and the nucleus depends on their distance from each other (Fig. 5.3).
The bremsstrahlung is much more penetrative when compared to the primary emitted electron. It is necessary to store the pure sources of β-radiation in containers with a low proton number in order to keep the amount of produced bremsstrahlung to a minimum. The intensity I- of a-monoenergetic electron beam decreases during its passage through an absorbent medium as described by the following relationship:
I = I0 .e-μd
where μ stands for linear attenuation coefficient, d for the thickness of the absorbent layer and I0 for the incident intensity. Linear attenuation coefficient μ (m-1) can sometimes be replaced by a mass attenuation coefficient μ .ρ-1, i.e. linear attenuation coefficient divided by the density of the absorbent medium. However in that case it is necessary to replace the thickness (d) with the „mass thickness“ of the absorbent medium R (kg . m-2). The equation (8.5) can then be expressed in the following way:
I = I0 .e-μR/ρ
The spectrum of β emitters is continuous. The maximum range is often described as the mass thickness of the absorbent medium that can completely absorb the emitted electrons. In human tissue ordinary β emitters have a range of millimetres.
Gamma radiation and its interaction with the electron cloud[edit | edit source]
Gamma radiation interacts with the absorbent matter indirectly through photoelectric effect, Compton scattering, and the creation of electron-positron pairs. The linear attenuation coefficient is composed of three main elements:
μ = τ + σ + χ
where τ stands for linear absorption coefficient for of photoelectric effect, σ for the linear absorption coefficient of Compton scattering, and χ represents linear absorption coefficient of the electron-positron pairs production.
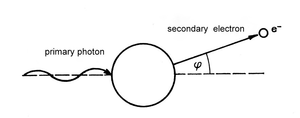
Photoelectric effect can be observed in cases when all of the gamma radiation energy is passed onto an electron from the electron cloud of the absorbent medium or onto a free electron, for example in metals. Part of the photon’s energy is used for liberating the electron (work function W) and the rest is transformed into kinetic energy of the newly produced photoelectron (see Fig. 8.4.). The photon of gamma radiation, which caused the photoelectric effect, disappears. Its energy is taken over by a photoelectron, which can then ionize its surrounding environment. The energy balance of this effect is described by the following Einstein’s equation:
E = h . υ = W + 1/2 m . v2
The atom that has lost the electron is in an excited state quickly transitions into the ground state through an emission of electromagnetic radiation. The frequency of the emission corresponds to the difference between the energy level of the excited and the ground state.
The probability of photoelectric effect interactions increases with the increasing atomic number of the absorbent medium. The probability is higher for gamma radiation with lower energy. For instance, when the absorbent medium is air, the photoelectric effect occurs only up to the energy levels of about 100 keV. For higher energy levels the probability of a photoelectric effect interaction is minimal.
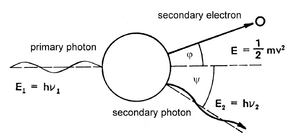
Compton scattering can be observed during the interaction of gamma radiation photons with higher energy levels. During this interaction, the gamma radiation photon passes only a part of its energy onto the absorbent medium electron. This electron then gains kinetic energy, and starts to move in a direction which is deviated from the course of the original primary photon by an angle φ. The secondary photon, characterised by a lower energy level (i.e. longer wave length) is deviated from the course by an angle ψ. The scattering of secondary photons fluctuates in the intervals between 0o and 180o. Energy levels of these photons depend on the angle of the scattering. For example the decrease of the primary photons energy level is the highest for the angle ψ = 180o (describing backscatter). The process can occur repeatedly until the photon loses so much energy that the probability of its destruction via photoelectric effect increases (Fig. 5.5). The energy balance of Compton scattering is:
hυ1 = hυ2 + 1/2 mev2
where me stands for the mass of the electron.
Compton scattering is important mainly in the case of gamma radiation with energy levels 0,5 up to 5 MeV. The scattering is practically independent on the proton number of the absorbent medium.
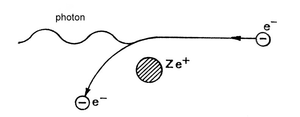
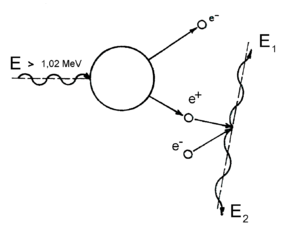
The production of electron-positron pairs (Fig. 8.6.) has its significance mainly in the case of gamma radiation with high energy levels, but also in the case of absorbent materials with high proton number. The energy of electromagnetic radiation in the vicinity of atomic nucleus or other particle can be completely transformed into electron and positron particles (with kinetic energies Ee and Ep). The presence of atomic nucleus or a third particle is necessary as they can take over a part of the photon’s energy. They can receive some energy because the sum of the momentum of produced electron-positron pair is smaller. The segregation of kinetic energies between the electron and the positron is random. The energy balance of this process is:
hυ = Ee + Ep + 2mec2
The energy balance equation states a clear condition under which this reaction can occur: The energy of the gamma radiation photon needs to be higher than the energy corresponding to twice the energy of the electrons rest mass (i.e. higher than 2 . 0,51 MeV = 1,02 MeV). In real life, this type of reaction occurs only upon reaching significantly higher energy levels. The newly produced electrons and positrons then continue to lose their kinetic energies through the process of ionization and excitation of the surrounding atoms. When the positron lowers its energy all the way down to values corresponding to the speed of thermal motion, it can be merged with a random electron. Their combined mass is then transformed into electromagnetic radiation energy. The most common result is two types of electromagnetic radiation quanta, each possessing the energy of 0,51 MeV. This represents the energy equivalent of the electron rest mass. Both of these quanta move in the opposite directions. The life length of the positron is about 10-7 s.
Fig. 5.6 Scheme depicting the origin of electron-positron pair
The probability of one of the above mentioned three possible gamma radiation interactions occurring, depends on the energy of the radiation. For lower energies the most common interaction is the photoelectric effect. For higher energies the most common interaction is Compton scattering, and for the highest energies the most probable interaction is the creation of electron-positron pairs. The gamma radiation ionizes the medium indirectly when passing through an absorbent medium. The ionization is due to the use of the secondary electrons produced by the interaction between gamma radiation and the environment. The measurable ionization loss of the gamma radiation is quite small. This is why the gamma radiation is much more penetrative than other types of radiation, and why shielding from it requires the use of materials with high proton number.
Neutrons and their interactions with the electron cloud[edit | edit source]
The primary interaction of the neutron radiation with matter is of a completely different kind than the previously described types of radiation. As particles without electric charge, neutrons do not have any influence on the electron field they are passing through. Their direct ionizing effects are completely negligible. The mechanism of energy transfer from the neutrons to the second medium lies completely within the area of nuclear reactions.
Links[edit | edit source]
Next chapter: 5.2.5 Interaction of the ionizing radiation with the atomic nuclei
Back to Contents