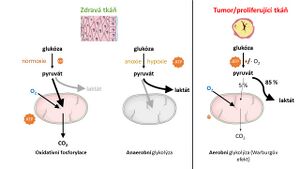
The Warburg effect
We use the term Warburg effect to refer to the specific metabolism of cancer cells that have less functional mitochondria and significantly reduced oxidative phosphorylation compared to healthy cells. Here, ATP synthesis is compensated glycolytically with concomitant lactate formation. The synonymous term aerobic glycolysis has been adopted for the Warburg effect.[1][2].
First half of the 20th century[edit | edit source]
In 1924, a German scientist described this tumour phenotype in somewhat simplified terms Otto H. Warburg[4]. Something similar was observed by Herbert G. Crabtree in yeast. He even confirmed that in these yeasts, when glucose or fructose is in excess, respiration is temporarily inhibited. Later he decided to test Warburg's claim not only on cancer cells but also on various tissues. Crabtree concluded his work by asserting that aerobic glycolysis is not the domain of malignant cells alone, but is inherent in any increased pathological proliferation[5]. Further experiments then led Crabtree to hypothesize that oxidative phosphorylation is crucial for the progression of specific tumors and that it is not possible to simplistically label the metabolism of tumor tissue as predominantly glycolytic[6].
Warburg's uniqueness lies in the fact that he was the first to understand the importance of oxidative and reductive metabolic pathways in living organisms and to put them in their proper context. He was awarded the Nobel Prize for his work in 1931 [1].
The hypothesis of non-functional or less functional mitochondria was confirmed only in 1951 by Feodor Lynen. By the 1950s, it was possible to quantify the rates of oxidative phosphorylation and glycolysis, which prompted Otto Warburg, then director of the Max Planck Institute, to write a new summary paper[7]. In it, he publishes a timeless hypothesis: "The energy itself will be at the center of our thinking." Even then, Warburg saw the weakness of cancer cells as using everything (not just glycolysis, but oxidative phosphorylation, for example) to meet the enormous need for ATP.
Late 20th and early 21st century[edit | edit source]
In the second half of the 20th century, a number of scientific groups were drawn to the possibility of using the Warburg effect as a target for anticancer therapy. The challenge was to selectively inhibit glycolysis and thus induce cell death. It turned out not to be that simple, although a number of pathological changes in the mitochondria of cancer cells have been identified[8]. It was not until 2004 that he contributed to the modern understanding of tumour metabolism Rodrigue Rossignol, by showing that the availability of energy substrates can alter not only the oxidative capacity of cancer cells but also the morphology of their mitochondria[9]. Further studies have shown that tumour cells work on the principle of the so-called metabolic remodeling. Many solid tumour cells proliferate very rapidly. At the same time, the malignant tissue does not vascularize fast enough. In similar cases, there is a transient lack of substrates of oxidative phosphorylation or glycolysis. Often there is also a reduction in oxygen concentration, which further complicates matters (see Reductive carboxylation)[10].
- ↑ SINGH, Keshav – COSTELLO, Leslie. Mitochondria and Cancer. - edition. Springer Science & Business Media, 2009. 289 pp. ISBN 9780387848358.
- ↑ WEINHOUSE, Sidney. On Respiratory Impairment in Cancer Cells. Science. 1956, y. 3215, p. 267-269, ISSN 0036-8075. DOI: 10.1126/science.124.3215.267.
- ↑ WANDER HEIDEN, MG et al.. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009, y. 324, p. 1029 - 1033, ISSN 0036-8075. DOI: 10.1126/science.1160809.
- ↑ Incomplete citation of article. WARBURG, Otto et al.. Metabolism of carcinoma cells. Biochem Z. 1924, y. 152, p. 309 - 344,
- ↑ CRABTREE, HG. The carbohydrate metabolism of certain pathological overgrowths. Biochem J. 1928, y. 22, p. 1289-98, ISSN 0264-6021. DOI: 10.1042/bj0221289.
- ↑ CRABTREE, HG. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929, y. 23, p. 536-545, ISSN 0264-6021. DOI: 10.1042/bj0230536.
- ↑ WARBURG, Otto. On the Origin of Cancer Cells. Science. 1956, y. 123, no. 3191, p. 309-314, ISSN 0036-8075. DOI: 10.1126/science.123.3191.309.
- ↑ DIAZ-RUIZ, R.. Tumor cell energy metabolism and its common features with yeast metabolism. Biochimica et Biophysica Acta (BBA). 2009, y. 1796, p. 252-265, ISSN 0304-4165. DOI: 10.1016/j.bbcan.2009.07.003.
- ↑ ROSSIGNOL, Rodrigue et al.. Energy Substrate Modulates Mitochondrial Structure and Oxidative Capacity in Cancer Cells. Cancer Research. 2004, y. 64, p. 985 - 993, ISSN 00085472. DOI: 10.1158/0008-5472.CAN-03-1101.
- ↑ SMOLKOVÁ, Katarína et al.. The Role of Mitochondrial NADPH-Dependent Isocitrate Dehydrogenase in Cancer Cells. International Journal of Cell Biology. 2012, y. 2012, p. 0 - 12, ISSN 1687-8884. DOI: 10.1155/2012/273947.
