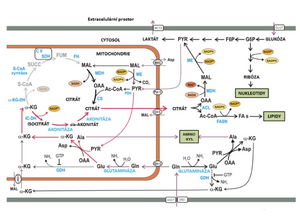
Reductive carboxylation
Reductive carboxylation (RK) represents a promising target for antitumor therapy and is thus still a subject of research. This is a metabolic pathway, the existence of which has also been proven physiologically. However, it seems to be particularly significant in tumor tissues. It plays an important role in adaptation to very low oxygen concentration.
Currently, a large number of scientific groups are engaged in cancer research, which agree that the metabolism of tumor cells works on the principle of so-called metabolic remodeling. All metabolic pathways in transformed cells are used for the production of ATP, building blocks and intermediates required for cell proliferation. In general, the modified pathways in tumor cells include glycolysis, the Krebs cycle, the pyruvate cycle (pyruvate carboxylation), lipogenesis, and the pentose-phosphate cycle.
Tumor cells proliferate rapidly and in the environment of a solid tumor, due to slow (insufficient) angiogenesis, they are forced to survive even in very low oxygen concentrations. RK is the reverse pathway of the Krebs cycle, in which isocitrate and citrate are formed from 2-oxoglutarate, and thus participates in the formation of precursors for lipid synthesis. The catalyst is NADPH-dependent isocitrate dehydrogenase 2 (IDH2). Template: Petit
In short, we can describe RC as follows:
- An important anaplerotic pathway of RK is glutaminolysis, which produces 2-oxoglutarate.
- 2-oxoglutarate is (reversed from the Krebs cycle) converted to isocitrate and further to citrate by the reverse aconitase reaction.
- Citrate can then be exported to the cytosol, where it is cleaved by ATP:citrate lyase to acetyl-CoA and oxaloacetate.
- Acetyl-CoA then serves as a precursor for the synthesis of fatty acids and cholesterol. Oxaloacetate can be further converted to malate, which is further metabolized.
RK following glutaminolysis was first described in brown fat, where up to 90% of glutamine is reductively processed and up to 40% of lipogenic acetyl-CoA originates from it. Subsequently, the relationship between RK and damage to mitochondrial oxidative metabolism was described. Similar to mitochondrial dysfunction, most citrate is reductively synthesized from 2-oxoglutarate in hypoxia, whereas glucose is the main source of citrate in normal cellular oxygenation. Activation of RK therefore occurs when mitochondrial oxidative phosphorylation is suppressed (by hypoxia or damage). The pathway makes it possible to maintain the level of citrate, which is necessary for the cell to divide, under conditions with a reduced possibility of substrate oxidation.
RK has also been described in human fibroblasts. The function of a kind of NADPH carrier (shuttle) is also attributed to it there. During RK, NADPH reduces 2-oxoglutarate, forming isocitrate and subsequently citrate. It is exported to the cytosol, where it can be reoxidized back to 2-oxoglutarate by the already mentioned IDH1, thereby producing cytosolic NADPH. As reducing equivalents do not readily pass through membranes, this is another elegant way of intercompartmental transhydrogenation.
Reductive carboxylation (RK) represents a promising target for antitumor therapy and is thus still a subject of research. This is a metabolic pathway, the existence of which has also been proven physiologically. However, it seems to be particularly significant in tumor tissues. It plays an important role in adaptation to very low oxygen concentration.
Currently, a large number of scientific groups are engaged in cancer research, which agree that the metabolism of tumor cells works on the principle of so-called metabolic remodeling. All metabolic pathways in transformed cells are used for the production of ATP, building blocks and intermediates required for cell proliferation. In general, the modified pathways in tumor cells include glycolysis, the Krebs cycle, the pyruvate cycle (pyruvate carboxylation), lipogenesis, and the pentose-phosphate cycle.
Tumor cells proliferate rapidly and in the environment of a solid tumor, due to slow (insufficient) angiogenesis, they are forced to survive even in very low oxygen concentrations. RK is the reverse pathway of the Krebs cycle, in which isocitrate and citrate are formed from 2-oxoglutarate, and thus participates in the formation of precursors for lipid synthesis. The catalyst is NADPH-dependent isocitrate dehydrogenase 2 (IDH2). Template: Petit
In short, we can describe RK as follows:
- An important anaplerotic pathway of RK is glutaminolysis, which produces 2-oxoglutarate.
- 2-oxoglutarate is (reversed from the Krebs cycle) converted to isocitrate and further to citrate by the reverse aconitase reaction.
- Citrate can then be exported to the cytosol, where it is cleaved by ATP:citrate lyase to acetyl-CoA and oxaloacetate.
- Acetyl-CoA then serves as a precursor for the synthesis of fatty acids and cholesterol. Oxaloacetate can be further converted to malate, which is further metabolized.
RK following glutaminolysis was first described in brown fat, where up to 90% of glutamine is reductively processed and up to 40% of lipogenic acetyl-CoA originates from it. Subsequently, the relationship between RK and damage to mitochondrial oxidative metabolism was described. Similar to mitochondrial dysfunction, most citrate is reductively synthesized from 2-oxoglutarate in hypoxia, whereas glucose is the main source of citrate in normal cellular oxygenation. Activation of RK therefore occurs when mitochondrial oxidative phosphorylation is suppressed (by hypoxia or damage). The pathway makes it possible to maintain the level of citrate, which is necessary for the cell to divide, under conditions with a reduced possibility of substrate oxidation.
RK has also been described in human fibroblasts. The function of a kind of NADPH carrier (shuttle) is also attributed to it there. During RK, NADPH reduces 2-oxoglutarate, forming isocitrate and subsequently citrate. It is exported to the cytosol, where it can be reoxidized back to 2-oxoglutarate by the already mentioned IDH1, thereby producing cytosolic NADPH. As reducing equivalents do not readily pass through membranes, this is another elegant way of intercompartmental transhydrogenation.
Therefore, the condition of RK is, on the one hand, the presence of IDH2, the so-called "interrupted" Krebs cycle (it refers to the reverse pathway 2-oxoglutarate - isocitrate - citrate) and the availability of substrates, including CO2. Some works state that a low concentration ratio of citrate and 2-oxoglutarate is crucial for the course of reductive carboxylation in mitochondria. Another factor necessary for the course of mitochondrial RK is the availability of NADPH – the direction of the IDH2 reaction is determined by the affinity for NADPH (Km for NADPH is lower than for NADP+) – and the presence of enzymes participating in RK.
Links[edit | edit source]
References[edit | edit source]
- DVOŘÁK, Aleš et al. Background Levels of Neomorphic 2-hydroxyglutarate Facilitate Proliferation of Primary Fibroblasts. Physiological Research. 2017, year. X, s. 293-304, ISSN 1802-9973.
- ↑ NAKAZAWA, Michael S. et al. Oxygen Availability and Metabolic Adaptations. Nat Rev Cancer.. 2016, year. x, s. 663-673, ISSN 1474-175X.
- ↑ ŠPAČKOVÁ, Jitka et al. Biochemical Background in Mitochondria Affects 2HG Production by IDH2 and ADHFE1 in Breast Carcinoma. Cancers. 04/2021, year. X, s. 1709, ISSN 2072-6694.
- ↑ TAVARERS, LC et al. Metabolic evaluations of cancer metabolism by NMR-based stable isotope tracer methodologies. Eur J Clin Invest. 2015, year. 45, s. 37-43, ISSN 1365-2362.
- ↑ MUZ, Barbara et al. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015, year. 3, s. 83–92, ISSN 2324-1128.
- ↑ SMOLKOVÁ, Katarína a Petr JEŽEK. The Role of Mitochondrial NADPH-Dependent Isocitrate Dehydrogenase in Cancer Cells. Int J Cell Biol. 2012, year. X, s. xxx, ISSN Hindawi.
- ↑ YOO, H. et al. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J Biol Chem. 2008, year. X, s. XXX, ISSN 0021-9258.
- ↑ MULLEN, AR et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012, roč. X, s. 385-8, ISSN 0028-0836.
- ↑ WISE, DR et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Natl Acad Sci U S A. 2011, year. x, s. 19611-6, ISSN 0027-8424 a 1091-6490.
- ↑ LEMONS, JMS et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol.. 2010, year. x, s. xxx, ISSN 1544-9173.
- ↑ SMOLKOVÁ, Katarína et al. Reductive carboxylation and 2-hydroxyglutarate formation by wild-type IDH2 in breast carcinoma cells. IJBCB. 2015, year. 65, s. 125-133, ISSN 1357-2725.
- ↑ GAMEIRO, PA et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 2013, year. 3, s. 372-85, ISSN 1932-7420 (online).
- ↑ SAZANOV, LA a JB JACKSON. Proton-translocating transhydrogenase and NAD- and NADP-linked isocitrate dehydrogenases operate in a substrate cycle which contributes to fine regulation of the tricarboxylic acid cycle activity in mitochondria. FEBS Lett. 1994, year. x, s. xxx, ISSN 0014-5793 (print); 1873-3468 (web).