The Fundamental Law of Radioactive Decay
- Previous chapter: 5.2 IONIZING RADIATION AND RADIOACTIVITY
Radioactive decay is an accidental process that occurs with a certain probability. It is impossible to predict exactly which nucleus is being transformed in a specific precise moment. However, if the number of radioactive nuclei is high enough, the process of radioactive transformation can be described mathematically. The total number of atoms (particles) N in the sample that have not yet been transformed at a time t, can be obtained from the following equation:
- N(t) = N0 . e-λt
(N(t) is the total amount of radioactive (untransformed) nuclei at a time t. N0 is the number of radioactive nuclei at a time t = 0. e stands for Euler’s number which is base of the natural logarithm – e = 2, 71).
The probability of a nuclear transformation is different for each radioactive nucleus and can be expressed by the decay constant λ. This is a constant of the proportion between the decrease of the number of radioactive nuclei (due to spontaneous transformation) and the total number of untransformed radioactive nuclei. The unit of the decay constant is s-1.
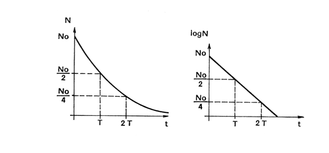
As it is shown in the radioactive decay equation (5.1), it is evident that the amount of radioactive nuclei decreases exponentially with time. This way of determining the amount of radioactive nuclei is not suitable for practical purposes and general use. By taking the logarithm of both sides of the equation we obtain the following expression:
- ln ( N(t) / N0 ) = – λt
The most suitable way to graphically express the equation 5.2 is to use a semi logarithmic graph (Figure 5.3).
- Next chapter: 5.2.2 Quantities and Units
- Back to Contents