The Compton effect - what does it consist of
Physical essence[edit | edit source]
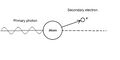
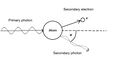
The Compton phenomenon (also referred to as Compton scattering, in English Compton scattering ) is a physical event that consists of the collision of atoms (electrons) with electromagnetic radiation. After the collision, due to the transfer of energy to the atoms (or their electrons) , the wavelength of the scattered radiation changes. The wavelengthλ of the scattered radiation is greater than the wavelength λ of the incident radiation. The frequency and energy of the scattered radiation are therefore smaller than the original values of the incident radiation.
The difference between the Compton effect and the photoelectric effect is that during the photo effect all the energy is absorbed, the photon disappears and only the so-called secondary electron is released (in this case it is called a photoelectron). In the Compton phenomenon, only part of the energy is consumed, so the photon does not disappear and a so-called secondary photon is released together with the secondary electron.
The equation of the Compton effect[edit | edit source]
Compton's equation gives the change in wavelength of a photon when it is scattered by an angle per particle with rest mass . Further is the speed of light and is Planck's constant. This change does not depend on the wavelength of the incident photon.
Greatness is called the Compton wavelength of the scattering particle, which for an electron is 2.4.10 -12 m. It can be seen from the relationship that the largest possible change in wavelength occurs at φ=180° when this change will be twice the Compton wavelength. The difference between the wavelength of the incident radiation and the wavelength of the scattered radiation is referred to as the Compton shift.
History[edit | edit source]
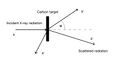
American physicist Arthur Holly Compton performed an X-ray scattering experiment on carbon in 1922. In 1927, he won the Nobel Prize in Physics for the theoretical explanation of this phenomenon and further research in this field. During his experiments, he let X-ray radiation with an energy of 17.8 keV fall on a carbon plate, which then scattered - the radiation was reflected in all directions. Compton measured the energy of the reflected photons as a function of the angle of reflection.
Explanation and significance of the discovery[edit | edit source]
An explanation of the Compton phenomenon is possible only with the help of the quantum hypothesis. Photons of X-ray radiation appear as particles when they collide with electrons in carbon. An elastic collision of a photon with speed c with a stationary electron occurs, and the law of conservation of energy and the law of conservation of momentum apply to this collision. This experiment thus became convincing proof of the quantum nature of electromagnetic radiation and proved the existence of photons (and their dual, wave-corpuscular character).
Links[edit | edit source]
Related articles[edit | edit source]
- Compton's phenomenon - what it proves and benefits
- Compton scattering
- Photoelectric phenomenon
- Wave-corpuscular dualism
References[edit | edit source]
- Navrátil L.; Rosina J. a kolektiv: Medicínská biofyzika, 1. vydání, Praha, Grada, 2005
- Amler E., Blažek T., Heřmanská J., Koláčná L., Kotyk A., Vackářová J., Varga F.: Praktické úlohy z biofyziky I. Ústav biofyziky UK, 2. lékařské fakulty, Praha, 2006
- Tarábek P., Červinková P. a kolektiv: Odmaturuj z fyziky, 2. vydání, Brno, Didaktis, 2006