Synthesis of Steroid Hormones
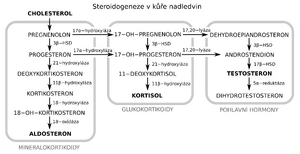
The starting point for the synthesis of steroid hormones is cholesterol. Its cyclopentanoperhydrogenphenanthrene core remains preserved, changes take place on the side chain:
Synthesis of Progesterone[edit | edit source]
Progesterone occupies a central position in the biosynthesis of steroid hormones.
- Oxidative degradation of the side chain – formation of pregnenolone
- Dehydrogenation – formation of progesterone (21 carbons)
Function[edit | edit source]
Progesterone is produced in the corpus luteum and the placenta. It ensures the transition from the tratum functionale of the uterus to the secretory phase (preparation for implantation of the fertilized egg), has antiestrogenic effects (reduces the sensitivity of the uterus to oxytocin, thus preventing premature birth). It causes thickening of cervical mucus (protection against spontaneous abortion), stimulates the growth of lobules and alveoli in the mammary gland. It is probably responsible for the rise in basal body temperature during ovulation. In large doses, it has a natriuretic effect.
Synthesis of glucocorticoids[edit | edit source]
It takes place in the adrenal cortex in the zona fasciculata. Successive oxidation by specific hydroxylases in position 17, 21, 11. The intermediate product 17α-hydroxyprogesterone is the starting point for the synthesis of sex hormones.
Cortisol = hydrocortisone - it is the most effective and affects the metabolism of carbohydrates and proteins.
Cortisone - is formed by dehydrogenation of cortisol - an oxo group is formed in position 11. It has slightly less effects.
Function[edit | edit source]
Glucocorticoids are in charge of mobilizing energy reserves, thus ensuring the survival of stressful situations and starvation.
- Action on carbohydrates: increase in blood glucose level - glucose utilization decreases, gluconeogenesis from amino acids increases.
- Effect on proteins: activation of catabolism - a negative nitrogen balance occurs.
- Action on lipids: lipolysis of fat deposits on the limbs and deposits on the abdomen and face - centripetal obesity.
- Other effects: reduction of the immune and inflammatory reaction, elimination of allergic processes, participation in the maintenance of blood pressure.
Mineralcorticoid synthesis[edit | edit source]
It takes place in the adrenal cortex in the zona glomerulosa.
Deoxycorticosterone (DOC) - hydroxylation occurs at the 21st carbon. It ensures the management of ions, but has a lower metabolic activity.
Corticosterone - DOC is hydroxylated at position 11β.
Aldosterone - The methyl group at position 18 is oxidized to an aldehyde group and a hemiacetal bond is formed between it and the hydroxyl group. Despite its low secretion, it is the most effective mineralocorticoid. It ensures the retention of Na+ and the excretion of H+ and K+.
Function[edit | edit source]
Mineralcorticoids ensure the management of water and ions. The target tissue is the distal parts of the nephron. They increase the resorption of Na+ and Cl– and subsequently the osmotic transport of water, resulting in an increase in plasma volume. They are also responsible for the increased excretion of K+ and also partly for the excretion of Mg2+. Potassium is excreted together with H+, and with increased secretion, the organism can reach a state of alkalosis. angiotensin II, reduced plasma volume (hypovolemia) and Na+/K+ ratio are the actual stimulus for the synthesis of mineralocorticoids.
Synthesis of male sex hormones[edit | edit source]
They arise from 17α - hydroxyprogesterone. A side chain is removed at carbon 17. It is produced in the testes and to a small extent in the zona reticularis of the adrenal medulla (by hydrogenation of androstenedione).
Synthesis of female sex hormones[edit | edit source]
It takes place in the follicles of the ovaries. The synthesis starts from testosterone, where the methyl group (carbon 19) is removed in position 10 and a compound with eighteen carbons - estrone - is formed.
Estrone - Contains an oxo group at position 17. Its effects are especially evident during menopause.
Estriol - It has a hydroxy group in position α16, it is less effective.
Estradiol - 3.17 β-estradiol.
References[edit | edit source]
References[edit | edit source]
- MUSIL, Jan – NOVÁKOVÁ, Olga – KUNZ, Klement. Biochemistry in pictures and diagrams. 1. edition. Avicenum, 1976. 368 pp. vol. 1.
- LEDVINA, Miroslav – STOKLASOVÁ, Alena – CERMAN, Jaroslav. Biochemistry for medical students, Part I. 1. edition. Karolinum, 2004. 274 pp. vol. 1. ISBN 80-246-0849-9.