Radioactivity (2. LF UK)
This article discusses a practical exercise in biophysics of the 2nd Faculty of Medicine, UK. If you want to know a bit more about the general theory of radioactivity, click here.
Theoretical basis[edit | edit source]
Radioactivity is a nuclear event in which an unstable parent radionuclide spontaneously changes into a more stable (again radioactive) or stable daughter nuclide that has a more optimal ratio of protons and neutrons in the nucleus. The purpose of radioactive transformation is to achieve stability of the atom. This event is characterized by the simultaneous emission of particles, quanta of electromagnetic radiation, or capture of an electron from the electron shell, producing ionizing radiation.
The process of radioactive transformation is repeated until the new nucleus is stable; consequently, its structure no longer changes spontaneously. Since these are processes taking place in the nucleus of an atom, it is not possible to influence the rate of transformation of the nucleus by any physical or chemical process. According to the different behavior of radiation in an electric and magnetic field, we distinguish radiation α, β and γ.
Radioactivity History[edit | edit source]
The discoverer of radioactivity is considered to be Henri Becquerel, a French physicist and member of the French Academy of Sciences, who initially dealt with the optical properties of substances. After the discovery of X-ray waves by the German physicist W. C. Roentgen in 1895, he began to focus on the relationship between X-rays and fluorescence, and while investigating the fluorescence of uranium salts, he came across a hitherto undescribed phenomenon - natural radioactivity. Experimenting with a photographic plate and a material that caused chemical reactions on the photographic plate without the need for light irradiation, he concluded that salts emit radiation of a non-light nature. He published the results of these experiments in 1896.
His discoveries were followed up by the spouses Pierre Curie and Marie Curie-Skłodowska. They discovered similar properties in 1896 of thorium compounds. Marie Curie-Skłodowska was the first to name this property, until then known as Becquerel rays, as radioactivity. After examining and measuring other rocks, the Curies discovered a number of other radioactive elements. These include polonium (150 times more radioactive than uranium) and radium (900 times more radioactive than uranium). In 1899, Marie Curie-Skłodowska supplemented her discoveries with the hypothesis that radioactivity is a natural process in which the nuclei of atoms of heavy elements emit radiation while simultaneously transforming into nuclei of lighter elements. This phenomenon was labeled natural radioactivity. Both husband and wife won the Nobel Prize together with Henri Becquerel in 1903.
The term artificial radioactivity was introduced in 1934 by a couple Frédéric and Iréne Joliot-Curie. They described this phenomenon on the basis of experiments with the bombardment of aluminum with alpha particles, during which a new phosphorus nuclide and a neutron were created. They won the Nobel Prize in 1935 for their experiments.
The discovery of radioactivity changed the world's view of the atom, which until then had been considered indestructible and immutable.
Laws of radioactive transformations[edit | edit source]
In a radioactive nuclide, emitting alpha or beta radiation, transformation processes take place, thanks to which a stable nuclide is formed. These can be characterized by activity A, specific or volume activities or according to the transformation half-time T1/2.
Activity A is expressed as the number of radioactive transformations per one a second. Measurement is in units of becquerel [Bq]. One conversion per second corresponds to 1 Bq. She used to be unit used is curie [Ci], 1Ci corresponds to 3.7*1010 Bq.
The law of radioactive transformation can be formulated mathematically as follows:
„The activity is directly proportional to the total number of nuclei not yet converted with the conversion constant.“
N corresponds to the number of atoms in the sample at a certain time t and λ is the conversion constant.
The law can be expressed in two other ways:
a) in exponential form
,
where N0 = number of atoms at time t = 0
b) in the differential state
From activity A we can express measured (mass) activity, which is equal to the activity of 1 kg of the emitter and volumetric (or surface) activity, which corresponds to the activity of one cubic (or square) meter of the emitter.
As the transformation half-time T1/2 we denote the time during which the activity of the sample drops to half. Since the discovery of radioactivity, 50 have been detected in nature natural radionuclides. Their half-lives take on different values on the order of seconds to billions of years. So clearly they can in nature be only those radionuclides that have a very long half-life, or those that they are constantly arising in nature.
Transformable The constant λ is characteristic of individual radioactive nucleus and expresses the probability of nuclear transformation. This constant corresponds to the proportionality between the number of converted radioactive nuclei and the total number of unconverted.
It can be expressed as follows:
Radioactive transformation is completely random, not possible can accurately determine the moment of transformation determine only the probability of a given transformation. Transformations are spontaneous without external intervention.
Types of ionizing radiation[edit | edit source]
α radiation[edit | edit source]
α radiation is a stream of flying helium nuclei so-called α-particles. An alpha particle consists of two protons and two neutrons, so it is positively charged with an electrical charge of +2e and has a non-zero rest mass.
The α radiation is produced by the radioactive transformation of isotopes of a heavy element, with the emission of an α' particle and the release of energy corresponding to the mass loss in the system.
The nuclide resulting from α decay has, due to conservation of nucleon number and electric charge, a proton number lower by 2, so it is moved two places to the left relative to the original nucleus in the periodic table of elements. Compared to the mass of the emitting nucleus, the emitted particle has a very small mass. The kinetic energy of the nucleus during the emission of the particle is practically negligible. The nucleus of the heavy element itself returns from the excited state to the energetically ground state by emission of γ radiation quantum. Therefore, it is common for this type of radioactive transformation to be accompanied by gamma radiation.
Alpha radiation has strong ionizing effects, but has low penetration. The propagation speed reaches up to 107 m.s-1 and the flying particle penetrates only a few centimeters of air. You can shade it with a regular sheet of paper. External effects on humans have practically no effect, as the radiation is absorbed by the cells of the squamous skin epithelium. However, the internal action of radiation (e.g. in the lungs) can damage the genetic material and thus lead to the development of cancer. Alpha radiation can also be used for medical purposes. Its action in certain doses activates the defense mechanisms of cells.
Examples of substances that are sources of α radiation include uranium, radium or radon.
β radiation[edit | edit source]
Beta radiation is a type of natural isobaric decay of radionuclides in which the number of nucleons in the nucleus remains unchanged. However, the nuclei of elements with an excess of neutrons emit particles carrying either a positive electric charge, called positrons (β+), or particles carrying a negatively charged electric charge, i.e. electrons (β-). In this process, an additional particle, the so-called antineutrino (v), is emitted which carries the difference between the energy released by the nucleus and the kinetic energy. The β radiation is about a hundred times more penetrating than the α radiation, but it has less ionizing effects. It can only penetrate low-density materials, or materials with a small thickness (even aluminium foil can trap it). Since it is an electrically charged particle, it is deflected in both electric and magnetic fields.
- β+: When an element emits a positron stream, a proton is converted into a neutron inside the original nucleus. As a result, the newly formed element has a proton number 1 unit smaller than the original atom, and the element shifts one place to the left in the PSP.
- β-: When a stream of electrons is emitted by an element, a neutron is converted into a proton inside the original nucleus. The proton number of the newly formed element is increased by 1 unit from the original atom, so the element moves one place to the right in the PSP.
γ-rays[edit | edit source]
Gamma radiation is electromagnetic radiation, unlike the previous two transformations. It has the shortest wavelengths (below 125-200 pm), extremely high wave frequencies (above 1019 Hz), and the highest energy (above 100 keV). It has a pronounced quantum impact, so it manifests as a stream of particles (photons). Photons are released from nuclei by rearrangement of nucleons in the radionuclide.
The energy of the nucleons in the nucleus is quantized; each nucleon occurs only in a particular quantum state. The transition from one state to another can only occur with the simultaneous delivery or release of energy. Thus, in γ-radiation, there is no transformation of elements, but only a reduction of the internal energy of the nucleons in the nucleus. The nucleus is created in an excited state and the excess energy is got rid of by emitting one or more photons of γ-rays.
Because of the very short wavelengths, γ-rays have high energy and high penetrability. The magnetic field does not affect the course of this radiation, since it is immaterial. γ-rays are usually accompanied by both α and β radiation.
Natural radiation background[edit | edit source]
Natural background radiation (also called natural radioactivity) can be characterized as ionizing radiation from space as well as our Earth. The value of this background is dependent on local conditions, but does not change significantly over time. The radiation itself has a random character and for specific places on the Earth's surface it acquires specific values that can differ greatly from each other, even more than a hundred times, which is mainly due to the different content of radioactive isotopes in the soil, the occurrence of radioactive sources created artificially by man and also different altitude.
Nature, together with all organisms, including humans, is well adapted to the current level of the natural radiation background. In addition, the human organism is able to gradually adapt to a higher value of the natural background during life.
The natural radiation background is therefore mainly caused by: - decays of unstable nuclei of radioactive isotopes in the earth's crust, possibly in her upper cloak - cosmic radiation and its interaction with the Earth's atmosphere
Protection against ionizing radiation[edit | edit source]
Due to the possible negative effects of ionizing radiation on the human body, three basic principles are used in practice for protection against this radiation: protection by time, protection by distance and protection by shielding. Their combination is also common.
You can find more about protection against ionizing radiation here.
Protection by shielding[edit | edit source]
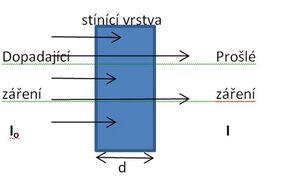
The principle of shielding protection against the effects of radioactive (ionizing) radiation consists in the use of an obstacle made of a certain material, which is placed between the radiation source and the expected location of the protected persons. Particles of ionizing radiation pass through the air relatively easily. However, if an obstacle made of material denser than air is placed in the path of these particles, the obstacle will absorb these particles (they will interact with each other) to an extent dependent on the thickness of the obstacle and its density. A barrier with greater thickness and density (higher proton number) shields radiation better.
The amount of absorbed (shielded) and the amount of transmitted radiation can be described by the exponential relationship I =I o . e -u . d (valid for a parallel beam of radiation), where I is the intensity of the transmitted radiation , Io is the initial radiation intensity, d is the obstacle thickness and u is the linear attenuation coefficient. The intensity of the transmitted radiation "I" therefore decreases exponentially with the increasing thickness of the shielding layer "d". Instead of the linear attenuation coefficient, it uses the so-called half-thickness (d1/2) of absorption, which is the thickness of the layer of the given material that weakens halve the intensity of the given radiation.
Weakening of radiation intensity by an absorbing (shielding) layer of thickness d can be expressed by the simple relationship I / Io = 2 -d/(d1/2) , where I is the intensity of the transmitted radiation, Io is the initial radiation intensity, d is the shielding thickness and d1/2 is the half-thickness absorption. From this relationship, we can easily calculate the total thickness of the shielding layer from a specific material for a certain desired specific value of radiation attenuation.
The following table lists the calculated thickness values of selected materials for certain values of gamma radiation attenuation.
| attenuation value I/Io for radiation with energy E = 200 [keV] |
concrete |
lead |
|
50% ( = half thickness) |
21 |
1,4 |
|
10% |
70 |
4,7 |
|
1% |
140 |
9,3 |
|
0,1% |
210 |
14,0 |
For example, it follows from the values that a radiation attenuation value of 10% requires shielding with a thickness corresponding to approximately 3.3 half-thicknesses, a radiation attenuation value of 1% requires a shielding with a thickness corresponding to approximately 7 half-thicknesses and a radiation attenuation value of 0, 1% requires shielding with a thickness corresponding to approximately 10 half-thicknesses (all calculated for a radiation energy value of E = 200 keV).
Note: From the above relationship I = I o . e - u . d might seem that the radiation cannot be completely shielded, since the basic property of an exponential function with a negative exponent is that it only approaches zero at infinity. In reality, however, each emitter emits only a finite number of quanta. After passing through a sufficient thickness (tens or hundreds of half-thicknesses d1/2) in practice, even the last quantum will always eventually be absorbed.
Practical use[edit | edit source]
For shielding protection in practical use, we need to identify the predominant type of radiation. With proper protection, the danger of radiation exposure can be significantly eliminated. We find important application of these measures in energy, construction and medicine.
For gamma radiation, elements with high density and high proton number must be used, most commonly lead (Pb). Typical examples are lead containers for storage and transport of gamma emitters or lead bricks. Concrete with admixture of barite (surrounding a nuclear reactor), as well as steel, tungsten or magnetite, are particularly suitable as building materials. These materials are added to exterior plasters and paints. In medicine, lead screens that block radiation from an X-ray machine are especially important. The lead cover does not have to be robust at all - a layer of a few millimeters, at most a few centimeters is usually enough to shield the radiation (it depends on the material, intensity and nature of the radiation). This is proof of another property of the named materials - they have relatively low half-thickness values.
Compared to gamma radiation, beta radiation is weaker and less penetrating, so protection against it is easier and less expensive. It is most often made of Plexiglas, aluminum foil or plastic. An important fact is that due to the occurrence of so-called bremsstrahlung radiation, we cannot use lead as a shield to protect against beta radiation.
Because of its low penetration and speed, alpha radiation can be easily shielded with just paper.
Practical tasks[edit | edit source]
All the values you measure are written into an excel table. This table is on the desktop in the "Representative-Czech Praktikum" folder, where you copy the "T7_Radioactivity.xls" table to the desktop, then rename it to "T7_Radioactivity_KruhSkupina". This spreadsheet automatically calculates averages and other data for you, and also generates graphs, making your work much easier. You only fill in the yellow boxes! After the measurement, save the completed table in the "Representative_České Praktikum" folder and the "Results" subfolder.
Gamabet Kit[edit | edit source]
For practical tasks, you will use a school kit with a Gamabeta radiator. The set includes shielding plates made of aluminum, iron, tin, copper and lead. The type of radiation that the device will emit can be set by turning the red mark on the emitter cover against the letter G or B. The parameters of the subsequently emitted radiation can be found in the table.
| Letter | Type of radiation | Nucleate | The half-life of a nuclide |
|---|---|---|---|
| G | gama | 241Am | 432,7 let |
| B | beta | 90Sr | 29,1 let |
Connect the pulse counter with a cable to the radiation indicator (light gray rectangle with a notch), turn on both devices. Do not place a replaceable absorber plate (metal prism; used in high activity radiation measurements) on the indicator.
Task #1: Measuring the radiation background[edit | edit source]
Since natural radioactivity occurs in small amounts all around us, the value of this background in the vicinity of the radiation source is determined in order to achieve a more accurate result when measuring the values of the detected particles coming from the source.
The measurement is performed 10x after 100 seconds, without using the radiation source, which you place as far as possible from the detector (outside the room) to make the measurement more accurate. From the measured values, the average value of the radiation background for 100 s is calculated, from which the resulting value for the ten-second interval is then determined.
Task #2: Shield Protection[edit | edit source]
The measurement is performed 5x after 10 seconds for 3 different metal absorption plates (Al, Cu, Fe), the emitter is placed 4 cm from the detector. The first series of measurements is for β radiation and the second for γ radiation. We change the type of radiation by turning the head of the emitter so that the symbol of the given radiation is on the side of the emitter with the hole.
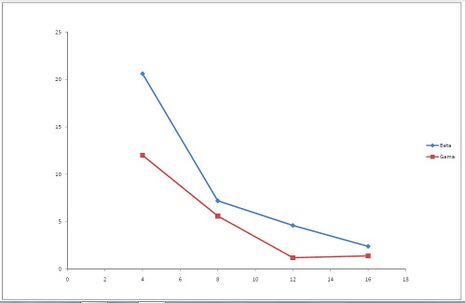
Task #3: Protecting Distances[edit | edit source]
The measurement is performed 5x after 10 seconds for 4 specified distances - once for β radiation and the second time for γ radiation. So - we measure the given radiation five times at a distance of 4 cm, five times at a distance of 8 cm and so on. Then we turn the head of the radiator and measure the second radiation in the same way. The value of the radiation background is subtracted from the obtained values in order to minimize the measurement error. The result of this task is a graph and your conclusions. You either support the graph with theory, or deny it with theory and explain why it does not correspond to theoretical knowledge.
For tasks 2 and 3, do not forget to secure the source in the groove with a stop, otherwise the measured values may be inaccurate.
Task #4: Poisson Distribution[edit | edit source]
This probability distribution, named after the French mathematician S. D. Poisson, has random variables that describe the frequencies of events with the following properties:
- the probability is proportional to the size of the interval (lambda for half the interval will be half)
- the probability that two events will occur at one moment (in one place) is negligible
- the probability does not change in different equally sized intervals
- the probabilities of events in different intervals are independent
(The last two assumptions are especially important. If they are violated, they bring additional dispersion (overdispersion) and we can easily recognize that the character no longer has a Poisson distribution)
then the probability distribution of the number of occurrences of a phenomenon in a certain time interval is called the Poisson distribution and has the probability density
p(x)=(λx/x!)*e-λ
in the given unit section, λ > 0 is a parameter, X =0, 1, 2,...
We denote the Poisson distribution by P(λ);
its mean value and variance (average number of occurrences of the investigated phenomenon in a given section of unit length) is equal to the constant λ
Furthermore, the occurrence of the observed phenomenon depends only on the length of the interval t and not on its beginning or on the number of times the phenomenon occurred before its beginning. This is called the Poisson distribution has no memory.
X = number of occurrences of a given random event per unit of time
However, if we were to repeat the measurement for the same time interval of length T, we would measure different numbers of X pulses. The reason is the statistical nature of the radioactivity. In doing so, we will measure some specific numbers of X more times than others. The probabilities that we measure a certain number of pulses X during the measurement time T differ from each other! However, mathematical statistics can determine and predict these probabilities (we are talking about probability distribution) E.g. a regular dice has an even distribution of probability, i.e. any of the numbers 1 to 6 must fall with equal probability.
When we have a large number of particle detection measurements from the emitter (in this case 50 measurements after 10 seconds for one type of radiation, when the emitter is 4 cm from the detector), we can construct a graph of the frequency of individual values. This is a "Histogram" - a simple evaluation of which value was recorded how many times.
The average of the measured values is automatically calculated - the so-called mean value a, according to which a graph of the theoretical distribution of values is drawn up. In short: the average number of recorded pulses (detected particles) has the highest frequency in the ideal state, the frequency gradually decreases towards a smaller and larger number of pulses.
The graphs obtained are then combined into one, so that the error caused by a small number of measurements can be corrected. The correction setting is done so that the Poisson curve better complements the experimentally measured values. The measured values of the highest frequency should correspond as closely as possible to the "peak of the curve". The added value "a" is the chosen average of the values, which shifts the ideal image of the graph to the real situation of the given measurement.
Empirical rules for choosing the Poisson distribution We use the Poisson distribution when
- variance is approximately equal to the mean,
- average is less than 10,
- the histogram of the data is skewed (the skewness disappears as the average increases, above 10 the histogram is almost symmetrical)
Terms[edit | edit source]
Finally, don't forget to write down the external physical conditions when performing the experiment (temperature, air humidity and air pressure). Although there is even a small amount of radioactivity in our surroundings, it is appropriate to write conditions for human protection against radioactive radiation in the conclusion. These conditions include, in particular, distance (the farther a person stays from the source of radioactive radiation, the more protected he is from higher radiation), time (the longer a person stays near the source radioactive radiation, the higher the chance of its exposure). For radiation workers, radiation protective clothing is used.
Resources[edit | edit source]
- ROSINA, Jozef. Biofyzika : pro zdravotnické a biomedicínské obory. 1. edition. Praha : Grada, 2013. ISBN 978-80-247-4237-3.
- SVOBODA, Emanuel. Přehled středoškolské fyziky. 4. edition. Praha : Prometheus, 2008. 0 pp. ISBN 9788071963073.
- AMLER, E. Praktické úlohy z biofyziky I. 1. edition. Praha : Ústav biofyziky UK, 2. lékařské fakulty, 2006.
- LUSTIG, F. – BROM, P.. Závislost radioaktivity na druhu a tlouštce vrstvy stínicího materiálu [online]. [cit. 2014-12-16]. <http://kdt-38.karlov.mff.cuni.cz/shielding/theory.html>.
- ŠVUB, M.. Poissonovo rozložení pravděpodobnosti [online]. [cit. 2014-12-16]. <https://is.muni.cz/th/rhxzm/poissonovo_rozlozeni_svub.pdf?so=nx>.
- ČEZ, A.S.,. Poznávej bez obav ionizující záření: souprava Gamabeta 2007 [online]. [cit. 2015-11-30]. <https://www.cez.cz/edee/content/file/pro-media-2014/05-kveten/gamabeta_letak.pdf>.
- BROKLOVÁ, Zdeňka. Učíme jadernou fyziku. [online]. [cit. 2012-02-29]. Available from: https://www.cez.cz/cs/vyzkum-a-vzdelavani/pro-studenty/materialy-ke-studiu/tiskoviny/11.html
- DUFKOVÁ, Marie. Domácí pokusy z jaderné fyziky. [online]. [cit. 2012-02-29]. Available from: https://www.cez.cz/cs/vyzkum-a-vzdelavani/pro-studenty/materialy-ke-studiu/tiskoviny/9.html
- Prepared measurement protocol 2014/2015
- Břetislav Fajmon, UMAT FEKT, VUT Brno Poissonovo a exponenciální rozdělení pravděpodobnosti