Quantitative analysis
Quantitative analysis (also titration determination, titration) generally belongs to quantitative methods that deal with determining the amount of individual components in the examined (analyzed) material (sample), which have already been determined in advance by qualitative analysis, determining only the composition of the analyzed sample.
Quantitative analysis is performed in a liquid environment, it belongs to direct chemical methods of analysis alongside instrumental analytical methods that use indirect physio-chemical measurements for quantitative determination.
Quantitaive analysis uses stoichiometric analytical reactions for quantitative determination. The condition is that such reactions take place unambiguously, quickly enough, without disturbing side reactions and quantitatively, with the possibility of easily identifying the end of the reaction, reaching the equivalence point.
The principle of titration is the precise measurement of the volume of the measuring (titration) reagent solution, which is gradually added (from the burette) to the precisely known volume of the analyzed sample solution (in the titration flask) until the moment when a quantitative chemical reaction takes place between them (equivalence point). According to the stoichiometric ratios of the chemical equation of the reaction, from the measured volume of the titrant used during the titration, from its concentration and from the volume of the solution of the analyzed sample, we calculate the equivalent amount of the analyzed substance in the volume of the analyzed sample or directly its concentration.
Titrations can be performed as:
- Direct titration, when a measuring solution is added directly to the solution of the substance that is determined until the moment when the substance amounts of both solutions are equivalent.
- Indirect titration, when an excess of reagent is added to a solution of the substance to be determined, a product is formed that is yet to be titrated.
- Back titration, when the exact volume of the measuring reagent in excess is added to the solution of the substance to be determined, a quantitative reaction takes place, then the excess of the measuring reagent is titrated with another measuring reagent.
Methods of quantitative analysis[edit | edit source]
The methods of quantitative analysis are divided according to the principle of chemical reactions, which are the essence of the titration determination, into:
- acid-base titrations (determination of acidic or basic substances by titration with bases or acids), the principle is neutralization reactions
- H3O+ + OH- 2 H2O
- complexing (titration, when the cation of the determined metal is bound to a soluble complex ion, so that it dissappears from the solution as free cation), viz complex compounds.
- precipitating (titration based on the formation of poorly soluble compounds, the titrated substance disappears from the solution because it tis precipitated drom it), viz solubility product. Eg. argentometry, which uses the formation of insoluble salts with the Ag+cation, the titration agent is AgNO3, suitable for the determination of Cl-, Br-, I-, CN-, SCN- and others.
- oxidation-reduction (the titrated substance is oxidized or reduced by an oxidizing or reducing titrant)
Invidual types of titration are also sometimes named according to the nature of the solution of the titrant (alkalimetry - we use a measured solution of a base, acidimetry - we use a measured solution of an acid, manganometry - we titrate with a measured solution of KMnO4, etc.). If the end the titration is determined by one of the instrumental indications, then this appears in the name of the titration, e.g. potentiometric, conductometric titration.
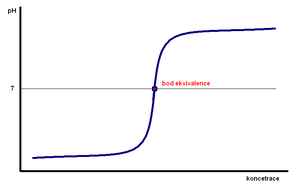
Titration curves[edit | edit source]
__
During acid-base (neutralization) titrations in aqueous solutions, during the titration of acids with bases or vice versa, the concentration of hydrogen ions in the titrated solution changes according to the nature of the ongoing equilibrium reactions of the titrated substances and titration reagents. pH can be calculated from their dissociation constants and the ionic product of water, or we can measure with a pH-meter throughout the titration (instructions). By graphically representing the dependence of pH changes on the amount of added titrant, we get a titration curve. E.g. if we titrate a solution of a strong acid with a strong base or vice versa, its salt is formed gradually until the moment when the titrated solution contains a neutral salt at the equivalence point, the pH will be 7. In the case of titration of a weak acid with a strong base due to the hydrolysis of the salt, the pH will be of the equivalence point shifted towards a higher pH value, in the case of titration of a weak base with a strong acid, analogously to lower pH values. It is clear from the dependences that the greatest change in pH occurs at the equivalence point, which is already used in the aforementioned indication of the equivalence point of acid-base chemical reactions during titrations. E.g. the measured dependences also show the difference between the titration and actual (actual) acidity of a weak acid, the titration acidities are approximately the same for a strong and weak acid, if they are of the same density and of the same concentration, while the actual acidity differs. Furthermore, we can subtract the pK of weak acids and bases from these dependencies.
Determination of equivalence point, indicators[edit | edit source]
In chemistry, the end of titration is reffered to as the equivalence point - the state when substance amount of the titrant (its substance concentration multiplied by the consumed volume during the titration) is equivalent to the substance amount of determined substance.
Methods of indicating the equivalence point[edit | edit source]
- Visual indication – subjectively observable change in the titrated sample solution, color change, formation of a precipitate, fluorescence. Most often, it is the change in the colour of a suitable dye, an indicator, added to the titrated solution, which occurs exactly when the equivalence point is reached.
- Instrumental indication – measured by machines, when one of the physical quantities of the titrated solution is measured (e.g.conductivity of the solution, pH, etc.) depending on the volume of the added titrant - again a titration curve. The quantity is chosen so that at the point of equivalence, there is a substantial change on this curve (eg. a break), so we determine the consumptionof the titrant from a certain point of this change.
Indicators are substances that react with either the analyte or excess titrant, and reacted and unreacted form have different colors. At the point of equivalence, one form is transformed into another. They are substances of similar nature like the titrant or the titrated substance. Depending on the character of the titrations, there are numbers of different types of indicators.
Acid-base indicators (for titrations of acid and base). They are weak organic acids and bases. The acidis form (Hlnd) that existts at a higher concentration of H+ (lower pH) has a different color than the base form (Ind-). The indicator acid and base together form a conjugate pair with the ionization constant KHInd.
- HInd H+ + Ind-
According to the ions concentration of the titrated solution, the concentration ratio of both forms is established - protolytic equilibrium
By mathematical modification (negative logarithm of these relations) we get the expression
In the titration determination, e.g. of an acid with a measured base solution, the titration solution is in the acidic form. Both form are actually present right at the equivalence point. Just beyond the equivalnce point, the acid form dissapears completely (reacts with an excess of basic titrant to form the basic form). From this is clear that the color of the indicator usually changes gradually within a certain small range around the equivalence point. The ratio of log HInd/Ind- can take values from approximately −1 do +1, therefore the pH range of color transitions of acid-base indicators (functional area) is usually up to 2 pH units.
Examples of acid-base indicators:
- Methyl orange, functional region pH 3,0–4,4; red – orange;
- Sodium 4-[4-(dimethylamino) phenylazo]benzen-sulfonate
- Methyl red, pH functional range is 4,4–6,2; red – yellow;
- 2-(4-dimethylaminophenylazo)benzoic acid
- Phenol red, pH functional range is 6,8–8,4; yellow – red;
- Phenolsulphoftalein, 3,3-bis(4-hydoxyphenyl)-sulfoftalide
- Phenolphtalein, pH functional range is 8,2–10,0; colorless – red-violet;
- 3,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranone
Indicators of complecometric titrations - metallochromic, are substance that form a complex with the determined metal ion. The complex with the metal is differently colored than the free form of the indicator. Before the equivalence point, only the complex form with metal is present. Just before the equivalence when free ions of the determined metal can no longer get into the solution, the complexing titrants begins to react with the metal ion that was bound with the indicator, thereby displacing the indicator in free form .
Examples of metallochromic indicators: erichrome black T (transitions from violet to blue), xylenol orange (from red or violet to yellow), murexide (from yellow or red to violet).
Precepitation titration indicators form colored precipitated or soluble color complexes, or they can cause a change in the color of the precipitate or solution at the equivalence point due to absorption on the particles of the precipitate or conversely, desorption (see argentometry, solubility product).
Indicators of redox reactions. Substance whose reduced form is different in color from the oxidized form are very often used. The first excess of oxidizing titrant just past the equivalence point will convert the reduced form to the oxidized form, (examples benzidine or dephenylamine - they change from colorless to blue). A number of redox indicators work irreversibly, like some of the colored substances (e.g. methyl red), which are oxidized by the first excess of the oxidizing agent, which results in the decompostion of the substance, which results in the decomposition of the substance, which is manifested by discoloration. However, the reduced color form cannot be recovered by reduction.
Preparation of measuring (titration) reagent[edit | edit source]
A solution of the measuring agent can be prepared with the exact concentration by accurately weighing the substance, dissolving it and adding water up to the mark in the measuring flask, if the substance is stable, standard and chemically pure. More common method of the preparation is to prepare a measuring solution with an approximate concetration and titrate with it an exact volume of a standard solution with the exact concentration that the measuring solution should have - the so-called standardization of titration solutions. Using this procedure, we determine the titration factor f of the measuring reagent as the ratio of the volume of the theoretical consumption of the measuring solution in ml and the actual consumption of the measuring solution in ml. Then, when calculating the concentrations of substances from the titration determination, we use the titration factor to adjust (multiply) the substance concentration of the titrant.
- If the solution is of exact concetration, f = 1.
- If the solution is more diluted, f is less than 1.
- If the solution is more concentrated, f is greater than 1.
Own titration[edit | edit source]
The burette is rinsed with distilled water and a measuring reagent of known concentration is poured into it using a funnel. The volume of the solution of the analyzed substance (sample) is precisely measured into a clean titration flask and a few drops of suitable indicator are added. The titration flask has to be shaked in circles and at the same time a small amount of measured solution is gradually released from the burette. Before the end of the titration, i.e. when a change in the titrated solution (color, turbidity, etc.) begins to be seen, we add the titrant very slowly (drop by drop) until the titrated slution changes (it is easy to "over-titrate", i.e. a larger volume of measuring reagent is used). The average consumption of the second and third titrations is used to calculate the concentrations. The colored solution is better observed against a white background, the resulting white turbidity, on the other hand, against a black background.
Calculation of substance and mass concentration from titration determination[edit | edit source]
When calculating the concentrations of substances determined by titration, we start from a state where a quantitative chemical reaction took place in a stoichiometric ratio, and at the equivalence point, the equivalent substance amounts of the measuring reagent and the titrated substance are equal. If X moles of titrant reacts with Y moles of titrant in solution, then the equation generally applies:
and if the product of concentration and volume is substituted for the amount of substance n, than the relation applies to:
| kde označuje | nx | substance amount of substance x (titrant) |
| ny | substance amount of substance y (titrated substance) | |
| X | number of reacting molecules of substance x | |
| Y | number of reacting molecules of substance y | |
| cx | concentration of substance x | |
| cy | concentration of substance y | |
| Vx | volume of solution of substance x | |
| Vy | volume of solution of substance y |
Example
For the titration of 10 ml of NaOH solution, 8 ml of sulfuric acid with an exact concentration of c = 0,1 mol/l. What will be the substance and mass concentration of the titrated NaOH solution?
Solution
A chemical equation applies to a titration
- H2SO4 + 2 NaOH Na2SO4 + 2 H2O
titrant H2SO4 (X = 1 mol), titrant NaOH (Y = 2 moly).
By modifying the above extract, we get
After reaching
The substance concentration of NaOH is cy = 0,16 mol/l.
Conversion to mass concentration: (Mr NaOH = 40)
mass concentration of NaOH is w = 6.4 g/l.
Links[edit | edit source]
Literature[edit | edit source]
- Karlíček R. a kolektiv (2001): Analytická chemie pro farmaceuty. Karolinum, Nakladatelství Univerzity Karlovy.
- Kraml J. a kolektiv (1999): Návody k praktickým cvičením z lékařské chemie a biochemie. Karolinum, Nakladatelství Univerzity Karlovy.
- Návod k použití laboratorního pH/mV/ORC-metru Orion 2 Star



![{\displaystyle K_{HInd}={\frac {[H^{+}]\cdot [Ind^{-}]}{[HInd]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/158cc7793b32f9a4fbb0ea01be836d164d5944af)
![{\displaystyle {\frac {[Ind^{-}]}{[HInd]}}={\frac {K_{HInd}}{H^{+}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5875863865c25f5d25e7d7167cf585e4e5db4e13)
![{\displaystyle pH=pK_{HInd}-log{\frac {[HInd]}{[Ind^{-}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b8f5053423e2f0dcfa7f0641178c20a5e755b032)






