Oxidation and the role of oxygen
Oxidation is the loss of electrons or the increase in the oxidation number of the respective element. The removed electrons must end up on another element, which is why the oxidation of one substance is always associated with the reduction of another substance. The word "oxidation" comes from the word "oxide", which refers to a compound containing oxygen. Oxygen is not absolutely necessary for oxidation to occur.
Oxygen is an oxidizing agent. It has a high tendency to accept electrons. The affinity of an element for electrons is expressed by the value of its electronegativity. The electronegativity of oxygen is one of the highest among the elements, only fluorine has a higher electronegativity. The transfer of electrons to oxygen, including the formation of bonds with oxygen, is a thermodynamically advantageous process, i.e. releases energy.
Organisms living on Earth have found a way to use energy by transferring electrons from less electronegative elements to oxygen. Our cells oxidize organic compounds contained in food to CO2' and H2O' for "oxygen consumption" and "energy production". This process is also sometimes called nutrient burning. Unlike combustion, the transfer of electrons from organic molecules to oxygen in our cells is divided into many steps. Most of the released energy is not converted into heat and light, but is stored in the form of a chemical potential. For didactic reasons, we can divide oxidative metabolism into two phases:
- Substrate oxidation associated with reduction of enzyme cofactors;
- Reoxidation of reduced cofactors by oxygen.
Breakdown of glucose[edit | edit source]
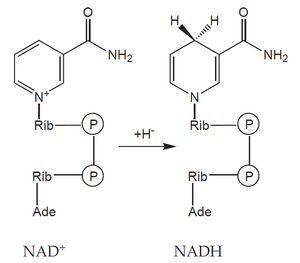
An example is glucose. Its six-carbon molecule (C6H12O6) is gradually oxidized into two molecules in the process of glycolysis and the pyruvate dehydrogenase reaction acetic acids (in the form of acetyl-coenzyme A) and two molecules of CO2. During this reaction, four molecules of the coenzyme NAD+ (nicotinamide adenine dinucleotide) are reduced to NADH, each accepting two electrons (often indicated as acceptance of the hydride anion H< sup>–).
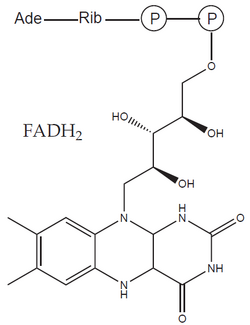
The formed acetyl-CoA enters the citrate cycle and is further oxidized to two molecules of CO2 and four molecules of reduced cofactors (three NADH and one FADH2 – flavin adenine dinucleotide). As a result, during the oxidation of glucose, 12 reduced cofactors'' and six molecules of CO2 are formed. No molecular oxygen (O2) was consumed in this process – the missing oxygen atoms were provided by water molecules. In it, oxygen is already present in a reduced form (O–II), which does not participate in electron transfer.
In order for the metabolic pathway to function, the reduced cofactors must be retroactively 'reoxidized. The process of reoxidation occurs most often in mitochondria, where reduced cofactors transfer the obtained electrons to oxygen (reduce it). The reduction of oxygen then leads to the release of a significant amount of energy.