Magnetic Spectrum
- Previous chapter: 5.1 ELECTRO-MAGNETIC RADIATION
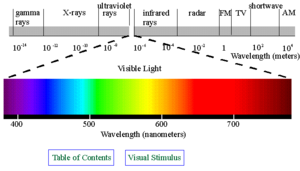
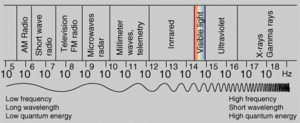
The brief account of familiar phenomena given above, surveyed electromagnetic radiation from small frequencies ν (long waves, radio waves) to exceedingly high values of ν (gamma rays). Going from the ν values of radio waves to those of visible light is like comparing the thickness of sheet of paper with the distance of the Earth from the Sun, which represents an increase by a factor of a million of billion. Similarly, going from the ν values of visible light to the very much larger ones of gamma rays represents another increase in frequency by a factor of a million of billion. This extremely large range of ν values, called the electromagnetic spectrum, is shown in the picture below (under construction), together with the common names used for its various parts, or regions.
The number ν is shared by both the classical and the modern interpretation of electromagnetic radiation. In classical language, ν is the frequency of the temporal changes in an electromagnetic wave. The frequency of a wave is related to its speed c and wavelength λ in the following way. If 10 complete waves pass by in one second, one observes 10 wriggles, and one says that the frequency of such a wave is ν = 10 cycles per second (10 hertz [Hz]). If the wavelength of the wave is, say, λ = 3 centimetres, then it is clear that a wave train 30 centimetres long has passed in that one second to produce the 10 wriggles that were observed. Thus, the speed of the wave is 30 centimetres per second, and one notes that in general the speed is c = λν. The speed of electromagnetic radiation of all kinds is the same universal constant that is defined to be exactly c = 299,792,458 metres per second. The wavelengths of the classical electromagnetic waves in free space calculated from c = λν are also shown on the spectrum in the picture below (under construction), as is the energy hν of modern-day photons. The common unit of energy is an electron volt (eV), which is the energy that can be given to an electron by a one-volt battery. It is clear that both the range of wavelengths λ and range of photon energies hν are equally as large as the spectrum of ν values.
Because the wavelengths and energy quanta hν of electromagnetic radiation of the various parts of the spectrum are so different in magnitude, the sources of the radiations, the interactions with matter, and the detectors employed are correspondingly different. This is why the same electromagnetic radiation is called by different names in various regions of the spectrum.
In spite of these obvious differences of scale, all forms of electromagnetic radiation obey certain general rules that are well understood and that allow one to calculate with very high precision their properties and interactions with charged particles in atoms, molecules, and large objects. Electromagnetic radiation is, classically speaking, a wave of electric and magnetic fields propagating at the speed of light c through empty space. In this wave the electric and magnetic fields change their magnitude and direction each second. This rate of change is the frequency ν measured in cycles per second, namely in hertz. The electric and magnetic fields are always perpendicular to one another and at right angles to the direction of propagation.
- Next chapter: 5.1.2 Generation of electromagnetic radiation
- Back to Contents