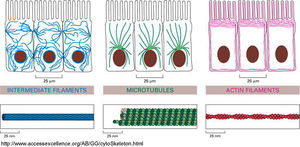
Individual filament structure and function
INTRODUCTION[edit | edit source]
Cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. It helps support the large volume of cytoplasm in a eukaryotic cell. It can be said that the cytoskeleton is not only the bones of a cell but also the muscles. The cytoskeleton takes credit for cell shape, motility (movement) of the cell as a whole, and motility of organelles within the cell. The cytoskeleton is composed by three types of protein filaments which are :
- Intermediate filaments
- Microtubules
- Actin filaments
Each of these are formed from a different protein subunit and have distinct mechanical properties. You can see further in the article the structure and function of these three filaments in more detail.
INTERMEDIATE FILAMENTS[edit | edit source]
STRUCTURE[edit | edit source]
A family of fibrous proteins form the intermediate filaments. The intermediate filaments are ropelike fibres with a diameter of about 10nm. The strands of the rope are elongated fibrous protiens. Each of these elongated fibrous proteins are composed of an N-terminal globular head, a C-terminal globular tall and a central elongated rod domain. The rod domain has an extended alpha helical region that enables pairs of intermediate filament proteins to form stable dimers by wrapping around each other. The central rod domains of different intermediate filament protiens have similar amino acid sequences and sizes. This ensures the formation of of similar diameter and internal structure when packed together. The globular domains vary greatly in the amino acid sequence and size from one filament protien to another. The filaments are given the name immediate because their diameter 10nm is between that of the thin actin-containing filaments and the thicker myosin filaments of smooth muscle cells. Intermediate filaments are the toughest and most durable amongst the three cytoskeletal filaments. While cytoplasmic intermediate filaments form rope like structures the intermediate filaments lining the inside of the nuclear membrane are organised as a two-dimensional mesh. These type of intermediate filaments are composed from a class of intermediate filament protiens called lamins. Intermediate filaments can be classed into four groups. Each group is formed by polymerisation of their corresponding protien subunits.
FUNCTION[edit | edit source]
Intermediate filaments have great tensile strength and their main function is to withstand mechanical stress occuringg from the stretching of the cells. Due to this function intermediate filaments are normally more prominent in the cytoplasm of cells more prone to mechanical stress. One of the places they are present in large numbers are along the length of nerve cell axons, where they provide essential internal reinforcement. They are also largely present in muscle cells and in epithelial cells of the skin. Intermediate filaments keep these cells and their membranes from breaking in response to mechanical shear, by stretching and distributing the effect of locally applied forces.
Medical signifiance of the functions of intermediate filaments[edit | edit source]
The rare human genetic disease epidermolysis bullosa simplex illustrates the importance of the intermediate filaments. In this disease mutations in the keratin genes interfere with the formation of keratin filaments in the epidermis, due to which the skin is highly vulnerable to mechanical injury, and even a gentle pressure can rupture its cells, causing the skin to blister. Mutations in the gene plectin cause a devastating human disease that combines epidermolysis bullosa simplex (caused by disruption of skin keratin), muscular dystrophy (caused by disruption of intermediate filaments in muscle), and neurodegenaration (caused by disruption of neurofilaments).
MICROTUBULES[edit | edit source]
STRUCTURE[edit | edit source]
Microtubules are long and relatively stiff hollow tubes of protien that can rapidly disassemble in one location and reassemble in another. They are built from molecules of tubulin, each one of which is itself a dimer composed of two very similar globular proteins called alpha tubulin and beta tubulin, and they are bound tightly together by noncovalentt bonding forming the wall of the hollow cylindrical microtubule. This tubelike structure is made of 13 parallel protofilaments,each a linear chain of tubulin dimers with alpha and beta tubulin alternating along its length. Each protofilament has a structural polarity, with alpha and beta tubulin exposed at two different ends, and this directional arrow embodied in the structree is the same for all protofilaments, giving a structural polarity to the microtubule as a whole. The beta tubulin end of the microtubule is called its plus end and the alpha tubulin end is called the minus end. A microtubule grows from an initial ring of 13 tubulin molecules. The tubulin dimers are added individually slowly building up the structure of the hollow tube. It is crucial for the structure of the microtubule to have a definite direction, with the two ends being chemically different and behaving differently. This is responsible for the assembly of microtubules and for their role once the microtubules are formed. If there was no polarity the microtubules could not serve their function in defining a direction for intracellular transport. The comparative instability of microtubules allows them to undergo continual rapid remodelling which is crucial for their functioning.
FUNCTION[edit | edit source]
One of the main function of a microtubule is to organise the interior of the cell. The cell's polarity is a reflection of the polarised systems of the microtubules inside, and this helps to position the organelles in the required location within the cell and to guide the stream of traffic moving between one part of the cell and another. Movement along microtubules is immeasurably faster and more efficient than free diffusion. The function of microtubules depend on a big variety of accessory protiens that bind to them. Microtubules are involved in saltatory and other directed intracelularr movements in eucaryotic cells. The movement is generated by motor protiens that bind to microtubules and use the energy derived from repeated cycles of ATP hydrolysis to travel steadily along the microtubule in a single direction. So microtubules and their respective motor proteins play and important part in positioning membrane enclosed organelles within a eukaryotic cells. Many microtubules in cells are stabalised through their association with other proteins and thats why they show no dynamic instability. Stable microtubules are employed by cells as stiff supports on which to construct a variety of polarised structures, including the cilia and flagella which help the eukaryotic cell to move water over their surface. The microtubules in cilia and flagella are a little different from the cytoplasmic microtubules.
Medical significance of the functions of microtubules[edit | edit source]
Cancer cells that divide with less control than most other cells of the body, can be kllled preferably by microtubule stabalising and microtubule destabalising antimitotic drugs. These drugs are derived from Colchicine and Taxol and are used in clinical treatment of cancer.
ACTIN FILAMENTS[edit | edit source]
STRUCTURE[edit | edit source]
Actin filaments are helical polymers of actin molecules. They are found in all eukaryotic cells. In electron micrographs actin filaments appear as threads that measure around 7nm in diameter. Each filament is a twisted chain of identical globular actin molecules, all these molecules point in the same direction along the axis of the chain. Therefore an actin filament has a structural polarity, with a plus and a minus end. Actin filaments are thinner,more flexible, and generally shorter than microtubules.There are many more individual actin filaments present in a cell than microtubules. So the total length of all the actin filaments in a cell is at least 30 times greater than the total length of all the microtubules. Actin filaments barely occur in isolation in the cell, they are usually found in cross-linked bundles and networks, which in turn are much stronger than the individual filaments. Actin filaments can grow by addition of actin monomers at either the plus or the minus end. The growth rate is faster at the plus end than at the minus end.
FUNCTION[edit | edit source]
Actin filaments allow animal cells to migrate. The function of actin filaments is dependant on the dynamic equilibrium between the actin filaments and the pool of actin monomers, and many filaments persist for only a few minutes after they are formed. A network of actin filaments underneath the plasma membrane forms the cell cortex and is responsible for the shape and movement of the cell surface, including the movements involved when the cell crawls along a surface. Actin rearrangements within the cortex provide the molecular basis for changes in cell shape and for cell locomotion.Many cell movements depend on the interaction of the actin and myosin filaments. If actin filaments and myosin filaments are organised together in a bundle, they can generate a contractile force. This is noticed most commonly in muscle contraction, but is also occurs in contractile bundles of actin filaments and myosin-II filaments that assemble transiently in non muscle cells. The contraction of a muscle cell is caused by the actin filaments sliding past the myosin filaments, with no change to their lengths.
REFERENCES[edit | edit source]
- ↑ Biology - Essential Cell Biology, 2nd Edition - (Bruce Alberts, Dennis Bray) Garland Science 2004
- ↑ http://www.nature.com/scitable/topicpage/microtubules-and-filaments-14052932
- ↑ http://www.biologyreference.com/Co-Dn/Cytoskeleton.html
- ↑ http://www.angelfire.com/sc3/toxchick/celmolbio/celmolbio14.html