Hemoglobin and its derivatives
Hemoglobin is a red blood pigment that transports oxygen from the lungs to the tissues and transports CO2 and protons from peripheral tissues to the respiratory system.
The hemoglobin concentration in a healthy adult male is approximately 150 g/l, in an adult female about 140 g/l. One gram of hemoglobin can bind up to 1.34 ml of oxygen.[1]
Hemoglobin:
Structure of hemoglobin[edit | edit source]
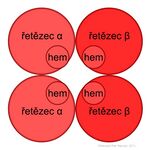
It is a tetrameric protein made up of four subunits. The two and two subunits are always identical. There are four types of polypeptide chains, physiologically occurring hemoglobin, α, β, γ, and δ, which differ in the number and sequence of amino acids. The tetramer consists of two α chains and two other types of chains that indicate the character of the whole hemoglobin molecule. In adults, hemoglobin A predominates, with two β chains (146 amino acids) involved in addition to two α chains (141 amino acids).
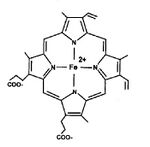
Each subunit includes a polypeptide chain to which one heme is covalently attached. The basis of the heme molecule is protoporphyrin, formed by 4 pyrrole nuclei connected by methenyl bridges with centrally bound iron. Heme iron is a total of six bonds - it is connected to the nitrogen atoms of the pyrrole nuclei by four coordination bonds. By another coordination valence, iron binds to the imidazole group of the amino acid histidine in the globin chain. The sixth valence Fe is for the oxygen molecule (O2).
Hemoglobin in the blood[edit | edit source]
Determination of hemoglobin in the blood is one of the most basic laboratory tests. Blood hemoglobin is the main criterion for assessing whether it is anemia. The term anemia is used when hemoglobin or erythrocytes fall below the lower limit of physiological levels. Anemia is a very common clinical finding. This is a condition that leads to a reduction in oxygen binding capacity and a consequent tissue respiratory disorder.
Causes of Anemia[edit | edit source]
Anemia occurs when erythropoiesis is unable to meet the requirements for new red blood cells. It develops as a result of blood loss or increased loss of red blood cells or insufficient red blood cell production. The following is a list of some specific causes of anemia:
- Anemia from increased blood loss:
- Acute blood loss.
- Chronic blood loss.
- Anemia due to increased erythrocyte breakdown (hemolytic conditions).
- Autoimmune hemolytic anemia (presence of antibodies against own erythrocytes).
- Erythrocyte membrane disorder (deviation in erythrocyte membrane composition).
- Hereditary erythrocyte enzyme defects (pyruvate kinase, glucose-6-phosphate dehydrogenase).
- Unstable hemoglobin - hemoglobinopathies (e.g. hemoglobin S in sickle cell disease).
- Anemia from decreased erythrocyte production:
- Lack of substances needed for erythropoiesis (iron deficiency, vitamin B12 deficiency, folic acid deficiency, erythropoietin deficiency - chronic renal diseases, lack of other substances such as vitamins B1, B6).
- Anemia due to chemical, physical and radiation damage.
- Anemia in chronic inflammatory, infectious and cancerous diseases.
Elevated hemoglobin levels may be a sign of dehydration or chronic decreased pulmonary ventilation. Rarely, it can be caused by some myeloproliferative conditions, such as polycythemia vera.
Principle of hemoglobin determination in blood[edit | edit source]
Oxidation of hemoglobin to methemoglobin:
| HbFeII | + | [FeIII(CN)6]3− | → | HbFeIII | + | [FeII(CN)6]4− |
| Hemoglobin | Methemoglobin |
Conversion of methemoglobin to cyanomethemoglobin:
| HbFeIII | + | CN− | → | HbFeIIICN | |
| Methemoglobin | Cyanomethemoglobin |
The photometric determination is based on the oxidation of ferrous iron in hemoglobin with potassium ferrocyanide to ferric iron. The resulting methemoglobin is converted to a very stable cyanomethemoglobin in a further reaction with potassium cyanide with a single broad absorption maximum in the visible region at 540 nm.
Assessment: The reference range for hemoglobin in the blood (B hemoglobin) for an adult male is 130-180 g / l and for a female 120-160 g / l.
Task: Determination of hemoglobin in the blood (pdf)
Hemoglobine in urine[edit | edit source]
Up to a million erythrocytes per day are excreted in the urine of completely healthy people. This very small amount cannot be demonstrated by conventional chemical tests. Occurrence of a larger number of erythrocytes (hematuria, erythrocyturia) or penetration of free hemoglobin, or muscle myoglobin, into definitive urine (hemoglobinuria or myoglobinuria) is almost always a pathological finding. We observe macroscopic hematuria with the naked eye; the urine is pinkish (comparable to water from washed meat) and hemoglobin can be detected spectroscopically in it. There is at least 1 g of hemoglobin per litre in the urine. In massive hemoglobinuria, the urine may have a colour of a dark beer (degradation of hemoglobin to hematin). Microscopic hematuria can only be detected biochemically.
Determination of hemoglobin in urine[edit | edit source]
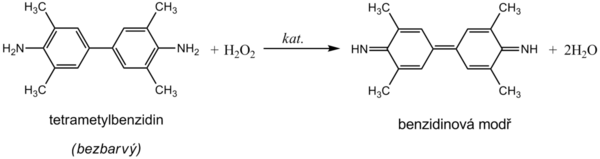
Hemoglobin catalyzes, like peroxidase, the oxidation (dehydrogenation) of some substrates (eg benzidine derivatives) by hydrogen peroxide:
However, it is not an enzyme activity (catalysis is conditioned by heme iron) and therefore it is not lost even after heat denaturation. We are talking about pseudoperoxidase activity, which is used for sensitive but non-specific evidence of hemoglobin or trace amounts of blood. It is preferable to use a chromogenic substrate to monitor the reaction, i.e., a substance that provides a markedly colored product by dehydrogenation (often benzidine or its non-carcinogenic derivatives, aminophenazone, etc.).
The reagent zone of the diagnostic stripes contains a chromogen (eg tetramethylbenzidine) with stabilized hydrogen peroxide (eg cumene hydroperoxide). In the presence of free hemoglobin (hemoglobinuria), the indication zone turns uniformly blue. If erythrocytes (erythrocyturia) are present in the urine, intensely green-blue dots to spots form.
Hemoglobinuria can be encountered in intravascular hemolysis. Damage to the glomerular membrane (glomerular hematuria) and bleeding from any part of the urinary tract lead to more frequent erythrocyturia. It is often found in urinary tract infections, urolithiasis and urogenital tract tumors.
In addition to hemoglobin, myoglobin also provides a pseudoperoxidase response, which can be excreted in the urine during skeletal muscle breakdown (rhabdomyolysis, crush syndrome). The positivity of the test may also be due to peroxidases of leukocytes or certain bacteria, yeasts or fungi, which may occur in the urine, especially in urinary tract infections. To rule out the possibility of a false-positive reaction due to cellular peroxidases, the reaction must be performed with boiled urine.
Contamination of the sampling vessel with strong oxidizing agents also causes a false-positive reaction. On the other hand, the presence of strong reducing substances (eg ascorbic acid) can slow down or even stop the pseudoperoxidase reaction and thus cause false-negative results.
Assesment: Determination of blood and hemoglobin in urine (pdf)
Hemoglobin in stool - occult bleeding[edit | edit source]
Demonstration of occult (hidden) bleeding is used to detect the early stages of colorectal cancer when radical and effective treatment is possible. The examination consists of capturing traces of blood in the stool, using various methodological procedures:
- The methods use the pseudoperoxidase activities of hemoglobin. The patient must maintain a diet for 3 days before the examination, exclude uncooked meat, salami, bananas, tomatoes from the diet, and must not take drugs containing ascorbic acid or acetylsalicylic acid. The patient then takes samples from three consecutive stools and applies them to the test cards. The evaluation is performed in the laboratory, the principle is similar to the hemoPHAN diagnostic stripes.
- Other methods are based on the immunochemical detection of hemoglobin with an anti-human hemoglobin antibody. Immunochemical methods are more sensitive and specific, there is no need to follow a diet before the examination. Positive results must be verified by other diagnostic methods.
Assessment: Test for occult bleeding in the digestive tract (pdf)
Hemoglobin derivatives[edit | edit source]
Hemoglobin derivatives include the following types:
Oxyhemoglobin and deoxyhemoglobin[edit | edit source]
Oxygen-carrying hemoglobin is referred to as oxyhemoglobin (oxy-Hb). Each Hb molecule can bind 4 molecules of oxygen. After the release of oxygen, we speak of deoxyhemoglobin (deoxy-Hb). In both forms, iron is divalent because only FeII + -containing hemoglobin can reversibly bind and transport the oxygen molecule. Oxygenation of the hemoglobin molecule changes the electronic state of the FeII + -hem complex, which results in a change in the color of the dark red (typical of venous blood) to a bright red color (arterial blood). In the human body, about 98.5% [2] of oxygen is bound to hemoglobin.
Carbaminohemoglobin[edit | edit source]
Carbaminohemoglobin is hemoglobin to which CO2 is bound. Carbon dioxide binds to the globin chain of hemoglobin. The binding of CO2 to hemoglobin reduces the affinity of hemoglobin for oxygen.
Methemoglobin[edit | edit source]
Methemoglobin (metHb; also hemiglobin or ferihemoglobin [1]) is characterized by the presence of ferric iron, which is formed by the oxidation of ferrous iron in hemoglobin [3]. Methemoglobin loses its ability to reversibly bind oxygen. In its place, FeIII+ binds a water molecule through the sixth coordination bond. The color of methemoglobin is chocolate brown. Methemoglobin is also present physiologically in small amounts in erythrocytes (about 1–3% of the total hemoglobin concentration [4]). This is mainly due to the effect of nitrite, which is formed from nitrates contained in food. The reverse reduction of methemoglobin to hemoglobin is mainly ensured by NADH-dependent cytochrome-b5 reductase (also methemoglobin reductase). A minor role is played by NADPH-dependent methemoglobin reductase, which is dependent on the supply of NADPH from the pentose cycle and on the presence of another electron transporter (eg flavin). [5] Non-enzymatic mechanisms include the action of glutathione and ascorbic acid.
Elevated blood levels of methemoglobin are called methemoglobinaemia. The causes are different:
- Hereditary methemoglobinemia is usually caused by a congenital defect of NADH-dependent methemoglobin reductase or the presence of abnormal hemoglobin M.
- Acquired methemoglobinemia is the most common form of methemoglobinemia. It may be caused by oxidizing agents [6]:
- poisoning by certain substances (nitrobenzene, aniline and its derivatives - eg some dyes),
- by the action of some drugs (local anaesthetics - benzocaine, then phenacetin, sulfonamides),
- increased content of nitrates and nitrites in water and food.
Newborns are particularly sensitive to the increased content of these substances due to the immaturity of the reduction systems and the increased proportion of fetal hemoglobin, which is more easily oxidized. Methemoglobinemia is manifested by cyanosis with a characteristic grey-brown tint and hypoxia.
| Methemoglobin values | Symptoms |
|---|---|
| 0–2 % | normal value |
| < 10 % | cyanosis |
| < 35 % | cyanosis and other symptoms (headache, dyspnoea) |
| 70 % | lethal concentration |
Part of the therapy of acquired methemoglobinemia is the administration of some reducing agents - methylene blue or ascorbic acid.
Carbonyl hemoglobin[edit | edit source]
Carbonylhemoglobin (COHb, carboxyhemoglobin) is formed by the binding of carbon monoxide to hemoglobin. The bond formed is 250-300 times stronger than the oxygen bond. Carbonyl hemoglobin cannot transport oxygen and cellular hypoxia develops due to the blood's reduced ability to carry oxygen. In excess oxygen, the binding of carbon monoxide to hemoglobin is reversible. Therefore, inhalation of O2 is most important in carbon monoxide poisoning.
COHb can also occur in small amounts in healthy people. For urban dwellers, values of around 2% are evident; for heavy smokers, COHb can rise to as much as 10% of total hemoglobin. Staying in an environment containing 0.1% CO for several minutes can increase the carbonyl hemoglobin concentration to 50%.
Carbon monoxide is formed during the imperfect combustion of fuels, it is also contained in exhaust gases and in smoke during fires in closed rooms.
| COHb values in % | Symptoms |
|---|---|
| 10 | more exertion shortness of breath |
| 20–40 | headache, shortness of breath, fatigue, vomiting |
| 40–60 | hyperventilation, tachycardia, syncope, convulsions |
| 60–80 | coma, death |
Carbonyl hemoglobin is crimson red; even people with severe carbon monoxide poisoning tend to have "healthy" pink skin. Compared to hemoglobin, carbonyl hemoglobin is more resistant to chemical influences, it changes more slowly due to the action of various agents.
Spectrophotometry of hemoglobin derivatives[edit | edit source]
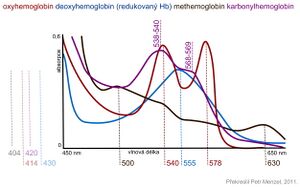
Hemoglobin and its derivatives have characteristic absorption spectra in the visible region of light, which are used for their analysis and rapid identification. Significant absorption maxima in the range of 400–430 nm, the so-called Soret band, are typical for all hemoproteins. Other absorption peaks are significantly lower. Oxyhemoglobin is characterized by two incompletely separated maxima in the region of 540 and 578 nm. Deoxyhemoglobin has a single absorption maximum at 555 nm. The main absorption maximum of methemoglobin is at 630 nm and the second faint peak at 500 nm depends on pH. By reacting methemoglobin with potassium cyanide, the maximum disappears at 630 nm, as cyanomethemoglobin is formed. The decrease in absorbance at 630 nm is proportional to the methemoglobin concentration. Cyanmethemoglobin has a broad absorption maximum at 540 nm, which is used to determine blood hemoglobin levels.The carbonyl hemoglobin spectrum is similar to that of oxyhemoglobin, but the position of the peaks is shifted toward lower wavelengths.
| Hemoglobin derivative | Absorption maxima [nm] |
|---|---|
| Hemoglobin reduced | 431, 555 |
| Oxyhemoglobin | 414, 540, 578 |
| Methemoglobin | 404, 500, 630 |
| Carbonylhemoglobin | 420, 538–540, 568–569 |
| Cyanmethemoglobin | 421, 540 |
Determination of carbonyl hemoglobin spectrophotometry:
Determination of carbonyl hemoglobin in the blood is one of the basic toxicological examinations. It is an objective criterion in the assessment of acute and chronic carbon monoxide poisoning.
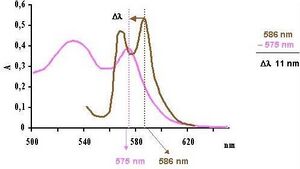
- Spectrophotometric evaluation. Carbonyl hemoglobin can be determined rapidly spectrophotometrically by subtracting the absorption maximum shift of the diluted blood from 586 nm [7]. The shift of the maximum in the spectrum depends on the ratio of COHb and O2Hb in the sample.
- Reactions with tannin. As a guide, carbonyl hemoglobin can be determined by reaction with tannin or Ajatin (about 10% COHb). Tannin forms a strawberry red precipitate in the presence of carbonyl hemoglobin. In the absence of carbonyl hemoglobin, the color of the precipitate is brownish grey.
- Acid-base balance analyzers. The analysis of the most toxicologically important hemoglobin derivatives COHb and metHb are also made possible by modern acid-base balance analyzers, which have a built-in photometric system for their measurement.
Task: Spectrophotometric examination of hemoglobin and its derivatives (pdf)
Task: Indicative determination of carbonyl hemoglobin (pdf)
Glycated hemoglobin HBA1[edit | edit source]
Glycated hemoglobin is formed by a non-enzymatic reaction between hemoglobin and blood glucose. Its creation is irreversible.
Glycated hemoglobin levels therefore reflect blood glucose levels throughout the life of the erythrocyte, i.e. about 120 days, and are used to assess the success of diabetes treatment / compensation in the 4-8 weeks prior to the examination. The form of the stable HBA1c fraction is most often determined.
Terminology
- Glycated hemoglobin - the sum of carbohydrate adducts at the N-terminus or ε amino groups of lysine in hemoglobin.
- HbA1 - the sum of various minor hemoglobin fractions (glycated), including HbA1c, HbA1a1 / a2, HbA1b1 / b2 / b3, HbA1d1 / d2 / d3 and HbA1e.
- HbA1c - glucose adduct of valine at the N-terminus of β-globin; corresponds to the so-called stable ketoamine (N- [1-deoxyfructosyl] hemoglobin).
Glycated hemoglobin can be determined by ion-exchange chromatography followed by spectrophotometry.
Evaluation
The amount of glycated hemoglobin is expressed in % of total hemoglobin or now in mmol/mol according to the IFCC (International Federation of Clinical Chemistry).
Reference limits
- in healthy adults up to 39 mmol/mol, (2.8–4.0%) [8]
- In diabetics, HbA1c concentrations of up to 45 mmol/mol (4.5%) indicate excellent diabetes compensation, up to 60 mmol / mol (6.0%) of acceptable and higher values of unsatisfactory diabetes compensation [9]
Task: Determination of glycated hemoglobin (pdf)
Iron[edit | edit source]
Iron is one of the most important elements in the human body. The adult body contains more than 70 mmol (4.0-4.5 g) of iron. In women, this amount is lower than in men, which is attributed to blood loss during menses.
| Form | Function | Protein | Amount in g |
|---|---|---|---|
| Active iron | oxygen transport | hemoglobin | 2,5–3,0 |
| myoglobin | 0,3 | ||
| electron transfer | cytochrome, cytochrome oxidase | 0,2 | |
| decomposition of hydrogen peroxide | catalase, peroxidase | ||
| Storage of iron | feritin, hemosiderin | 0,8–1,0 | |
| Trasport of iron | transferin | 0,003 | |
Iron metabolism[edit | edit source]
The presence of iron is essential for cell function. As a part of heme it participates in the transport of oxygen and as a part of cytochromes, it conditions the transfer of electrons in the respiratory chain. An undesirable effect of iron as a transient and highly reactive element is its participation in radical reactions, in which so-called reactive oxygen species are formed. These processes can damage cell membranes, proteins and DNA.
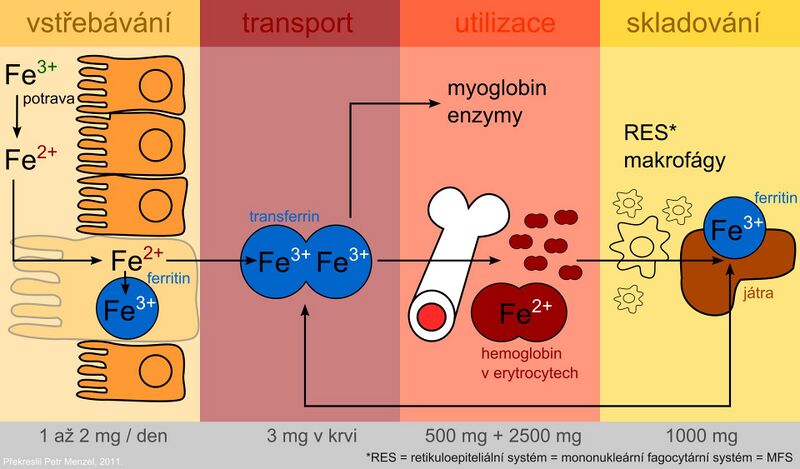
Iron is absorbed as Fe2+ by active transport in the duodenum and upper jejunum in two ways:
- porphyrin-bound Fe in the form of a stable lipophilic complex
- Water-soluble FeII+ chelates
Only a small part is absorbed in ionized form.
The diet averages 10-50 mg of iron per day, but only 10-15% is absorbed. Heme (meat) compounds absorb better, non-heme Fe in the plant diet is much worse. In addition, plants contain oxalates, phytates, tannins and other phenolic compounds which form insoluble or chelated complexes with Fe which are difficult to absorb. Ascorbic acid, on the other hand, improves iron absorption.
After uptake by the intestinal mucosa, part of the iron is incorporated into the storage form - ferritin in intestinal cells. Part of the absorbed iron passes into the plasma, where it is transported in binding to transferrin. The protein ferroportin (it is also found in the membrane of macrophages and hepatocytes) plays an important role in the transfer of iron across the basolateral membrane of enterocytes. It is the main site of regulation of iron homeostasis in the body. A key regulatory factor is the hepcidin protein, which is synthesized in the liver. By binding to ferroportin, it inhibits the transport of iron from cells and thus contributes to its sequestration in them. Hepcidin levels increase with inflammation. Hepcidin is also partly responsible for anemia of chronic diseases. Mutations in the hepcidin gene lead to juvenile hemochromatosis type 2B.
Plasma iron is captured by target tissue cells via the transferrin receptor and is either incorporated into heme or stored in ferritin. The use of the specific transport protein transferrin and the ferritin storage protein for iron storage represents protective mechanisms to prevent the toxic effects of redox-active iron.
During the desquamation of dead mucosal cells, unused iron leaves the stool together with unabsorbed iron.
Examination of iron metabolism[edit | edit source]
In practice, we commonly encounter diseases associated with changes in iron metabolism and utilization. Laboratory tests of iron metabolism include the following tests:
- iron in serum
- serum transferrin and iron-binding capacity
- serum ferritin
- transferrin receptor
These parameters are important diagnostic indicators for demonstrating a decrease or increase in iron stores even in stages that are not accompanied by significant clinical manifestations.
Determination of iron in serum[edit | edit source]
Colourimetric methods, atomic absorption spectrophotometry and other special techniques are used to determine serum iron. The most used are photometric methods based on the reaction of iron with a complexing agent. All procedures include the following steps:
- Release of Fe3+ from transferrin binding by acids or surfactants (eg HCl).
- Reduction of Fe3+ to Fe2+, which is necessary for the reaction with the complexing agent. Ascorbic acid, for example, is used for the reduction.
- Reaction of Fe2 + with a complexing agent containing reactive groups –N = C – C = N– to form a color complex. Metal ions form chelates with two nitrogen atoms. Currently, mainly two complexing agents are used - bathofenentroline and ferrozine (3- (2-pyridyl) -5,6-bis (4-sulfophenyl) -1,2,4-triazine - PST, protected name FerroZine®), which has a higher absorption coefficient and is more soluble in water.
- Evaluation
- Serum iron concentrations are subject to circadian rhythm and are affected by other factors. This limits the diagnostic significance of this parameter. It is a poor indicator of tissue iron stores and should always be considered in combination with serum transferrin and iron-binding capacity. Decreased concentrations are accompanied by iron deficiency, caused for example by large or repeated blood loss, insufficient dietary iron intake or impaired absorption. The finding is not specific, as reduced levels are also encountered in acute infections or chronic inflammatory diseases (iron transfer to tissues). High iron levels occur in hemochromatosis (see below), in iron overdose or intoxication, in increased erythrocyte breakdown, and in some liver diseases.
- Reference values
- men: 9–29 μmol/l
- women: 7–28 μmol/l
Serum transferrin and iron-binding capacity[edit | edit source]
Iron is transported by the blood in binding to a specific protein with β1-electrophoretic mobility - transferrin, which is synthesized in the liver. The rate of its formation is inversely proportional to the body's iron stores; it increases with iron deficiency and decreases with excess. The biological function of transferrin is the ability to easily form non-toxic iron complexes and transfer Fe absorbed by the small intestinal mucosa to the bone marrow or storage forms (ferritin or hemosiderin). Each transferrin molecule binds two Fe 3+ atoms (1 g transferrin binds 25.2 μmol iron). Transferrin can be determined directly by immunochemical methods or indirectly as the ability of transferrin to bind iron - the so-called iron-binding capacity. Total iron-binding capacity (TIBC) is the amount of iron that transferrin is able to bind when all binding sites are occupied. Usually, only 1/3 of transferrin-bound capacity is saturated with iron. Free transferrin without bound iron represents the free binding capacity (2/3 of transferrin) that is available for iron transport in increased requirements.
Conversion between transferrin concentration and total binding capacity:
- Total binding capacity [μmol/l] = transferin [g/l] · 25,2.
The reference range for serum transferrin concentration (S-transferrin) is 2.0–3.6 g/l and for total binding capacity is 50–70 μmol/l.
Transferrin saturation[edit | edit source]
From the values of iron and transferrin concentration, we can calculate transferrin saturation (TfS), which is defined as the ratio of serum iron concentration to total iron transfer capacity for transferrin. This is a sensitive parameter for detecting latent iron deficiency.
- Evaluation of transferrin saturation
- physiological values: 25-50% iron deficiency saturation reduction: <15% increase in saturation with excess iron:> 50%
Feritin and hemosiderin[edit | edit source]
Ferritin is the most important storage protein for iron. The ferritin molecule is adapted to bind large amounts of Fe3+ in a soluble and non-toxic form to the body. Ferritin consists of an outer protein shell of 24 subunits - apoferritin (Mr 440,000), delimiting a cavity in which up to 4500 iron atoms in the form of ferric oxyhydroxide (FeO · OH) n in microcrystalline form with phosphates (FeO · OPO3H2) can be concentrated. The entry and exit of iron atoms are allowed by the pores between the individual subunits of the ferritin shell. Normally, its capacity is used by about 20%. It is stored in cells in the liver, spleen and intestinal mucosa.
Ferritin is found in very low concentrations in the blood serum. Serum ferritin concentrations are a measure of the body's iron stores. Low concentrations indicate depletion of the body's total iron reserve and serve to detect iron deficiency anemia early in the prelatent phase. Elevated ferritin concentrations are an accompanying phenomenon of high tissue iron stores. We also encounter them in many patients with liver disease, some malignancies (tumor marker) or inflammatory diseases (acute phase positive reactant).
The reference range for serum ferritin (S-ferritin) is 30-300 μg/l for men and 20-120 μg/l for women.
Hemosiderin is another storage protein for iron. It is formed by the aggregation of denatured ferritin with other components. It forms particles with a size of 1 to 2 μm, which are visible in a light microscope when iron staining is used. Hemosiderin contains more iron than ferritin but is difficult to obtain due to its poor water solubility. It is formed when the amount of iron in the body exceeds the storage capacity of ferritin.
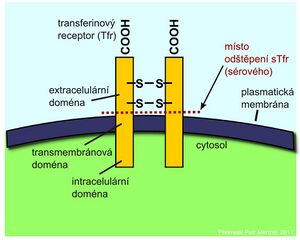
Transferrin receptor[edit | edit source]
Iron transported by blood by transferrin is taken up by cells via a specific transferrin receptor (TfR). It is on the surface of all cells at some stage of development but is most expressed on the surface of red cell precursors in the bone marrow. TfR is a transmembrane protein that is made up of two identical disulfide-linked subunits. By separating the extracellular domains of the receptor, the so-called soluble fraction of the transferrin receptor (sTfR), which may be in the form of a dimer or monomer, is released into the circulation. Cells respond to reduced iron stores by synthesizing increased levels of transferrin receptors.
An increase in sTfR is a reliable indicator of iron deficiency for hematopoiesis. Elevated sTfR levels are found in iron deficiency anemias or hemolytic anemias. Determination of sTfR is valuable in anemic patients in whom ferritin is elevated due to an acute phase reaction. Determination of sTfR levels can also be used in bone marrow transplant patients to monitor the course of erythropoiesis.
Immunochemical methods are used for the determination.
Disorders of iron metabolism[edit | edit source]
Iron deficiency in the body is usually caused by insufficient absorption from the intestine or by chronic blood loss. It can result in sideropenic anemia (hypochromic microcytic anemia), which is one of the most common hematological diseases. However, anemia is usually a late symptom of progressive sideropenia. It does not appear in the blood picture until the iron has almost completely disappeared. Therefore, it is necessary to detect iron deficiency at an early stage, which is not yet accompanied by anemia.
Based on the determination of the basic parameters of iron metabolism, we distinguish three degrees of deficiency:
- Prelatent iron deficiency is a sign of a state where there is a gradual decline in stocks, but the supply of iron to the bone marrow erythroblasts is not yet affected. In about half of the patients, serum ferritin levels are reduced below 12 μg / l.
- With latent iron deficiency, its reserves are essentially depleted. Ferritin is reduced below the lower limit of normal and is already accompanied at this stage by a reduction in serum iron levels and a reduced supply to bone marrow erythroblasts. The binding capacity for iron increases. A sensitive indicator of latent iron deficiency is a decrease in transferrin saturation below 15%. However, anemia is not developing yet.
- With manifest iron deficiency, anemia develops with hemoglobin levels falling below the lower limit of normal. Iron deficiency anemia is characterized by low serum iron and ferritin, and an increase in transferrin concentration (iron-binding capacity). In contrast, in hemolytic anemias or in excess of iron, serum iron is increased, and at the same time the total iron-binding capacity is reduced.
| Prelatent iron deficiency | Latent iron deficiency | Manifest iron deficiency |
|---|---|---|
| reduction of storage iron - decrease of ferritin | lack of storage iron - decrease of ferritin | lack of storage iron - decrease of ferritin |
| serum iron reduction | serum iron reduction | |
| decrease in transferrin saturation below 15% | decrease in transferrin saturation below 10% | |
| increase the total binding capacity for iron | increase the total binding capacity for iron | |
| increase in sTfR | increase in sTfR | |
| reduction in hemoglobin - anemia |
Excess of iron[edit | edit source]
The body is not equipped with an excretory pathway for iron, and therefore, under certain circumstances, excess iron may accumulate in the tissues. Early diagnosis can prevent tissue damage from excess iron. Iron overload usually develops very slowly. We distinguish 3 stages:
- In the stage of prelatent iron surplus, it's content in the organs increases, but without exceeding their storage capacity.
- During the latent stage of iron overload, the storage capacity of the cells is exceeded, but the function of the organs is not yet impaired, the ferritin level and the serum iron level increase and the transferrin saturation rises above 55%.
- In the phase of manifest iron surplus, some organs are already damaged.
| Prelatent iron excess | Latent iron excess | Manifest iron excess |
|---|---|---|
| increase in iron reserves - increase in ferritin | increase in iron reserves - increase in ferritin over 300 μg/l | increase in iron reserves - increase in ferritin (in case of severe impairment above
2000 μg/l) |
| increase in serum iron | marked increase in serum iron | |
| increase in transferrin saturation above 55% | increase in transferrin saturation (may exceed 90% in severe disabilities) |
Hemochromatosis
The accumulation of iron in the tissues is related to a disease we call hemochromatosis.
- Primary hemochromatosis is an inherited disease caused by increased resorption of iron in the gut. Excess iron is stored in parenchymal organs such as the liver, heart, pancreas, adrenal glands. It is toxic to the affected organs and disrupts their function by catalyzing chronic reactions leading to the formation of free radicals. The main clinical manifestations are skin hyperpigmentation, hepatosplenomegaly and diabetes mellitus.
- Secondary haemochromatosis may develop as a result of, for example, repeated transfusions, excessive iron intake or haemolytic anemia. In the biochemical picture, we find increasing levels of ferritin and iron in the serum, the saturation of transferrin increases with its simultaneous decrease.
Iron poisoning[edit | edit source]
Children are at risk of accidental ingestion of large quantities (by eating tablets similar to lentils). The lethal dose for a child is 600 mg. For an adult, iron intake of 40 mg / kg is toxicologically severe, and intake of 60 mg/kg is fatal [10].
Symptoms include nausea, vomiting (including vomiting blood), abdominal pain, diarrhoea (sometimes bloody). Large fluid losses cause shock, kidney failure and death. If the patient survives this phase of poisoning, he may become unconscious, convulsive and have failed after 12 hours. If he survives this second phase, the poisoning can have permanent consequences (intestinal damage).
- Treatment of acute poisoning
- Gastric lavage.
- Use the nasogastric tube to deliver the chelating agent deferoxamine. State Drug Control Office: deferoxamine (5-10 g to 50-100 ml water).
- Consider intravenous administration of desferoxamine to release absorbed iron. A pinkish-red complex of deferoxamine with iron appears in the urine. Treatment should be repeated until urine returns to normal [10].
Task: Determination of Fe in serum by colorimetric method (pdf)
References[edit | edit source]
Related articles[edit | edit source]
Reference[edit | edit source]
- ŠVÍGLEROVÁ, Jitka. Hemoglobin [online]. Poslední revize 2009-02-18, [cit. 2010-11-11]. <https://web.archive.org/web/20160416205421/http://wiki.lfp-studium.cz/index.php/Hemoglobin>.
- ↑ KITTNAR, Otomar a ET AL.. Lékařská fyziologie. 1. vydání. Praha : Grada, 2011. 790 s. s. 131. ISBN 978-80-247-3068-4.
- ↑ ŠVECOVÁ, D a D BÖHMER. Vrozená a získaná methemoglobinémia a ich liečba. Časopis lékařů českých. 1998, vol. 137, s. 168-170, ISSN 1803-6597.
- ↑ RICHARD, Alyce M, James H DIAZ a Alan David KAYE. Reexamining the risks of drinking-water nitrates on public health. Ochsner J [online]. 2014, vol. 14, no. 3, s. 392-8, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4171798/?tool=pubmed>. ISSN 1524-5012.
- ↑ XU, F, K S QUANDT a D E HULTQUIST. Characterization of NADPH-dependent methemoglobin reductase as a heme-binding protein present in erythrocytes and liver. Proc Natl Acad Sci U S A [online]. 1992, vol. 89, no. 6, s. 2130-4, dostupné také z <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC48610/?tool=pubmed>. ISSN 0027-8424.
- ↑ CORTAZZO, Jessica A a Adam D LICHTMAN. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth [online]. 2014, vol. 28, no. 4, s. 1043-7, dostupné také z <https://www.ncbi.nlm.nih.gov/pubmed/23953868>. ISSN 1053-0770 (print), 1532-8422.
- ↑ LEDVINA, M. Rychlé spektrofotometrické stanovení karbonylhemoglobinu v krvi. Biochem Clin Bohemoslov. 1987, vol. 16, s. 493-495, ISSN 0139-9608.
- ↑ ČEŠKA, Richard a Tomáš ŠTULC, et al. Interna. 2. vydání. TRITON, 2022. 870 s. ISBN 978-80-7387-885-6.
- ↑ Doporučený diagnostický a léčebný postup pro všeobecné praktické lékaře. Diabetes mellitus. 2005. Dostupné také z URL <https://www.svl.cz/files/files/Doporucene-postupy-2003-2007/Diabetes-mellitus.pdf>.
- ↑ Skočit nahoru k:a b ŠEBKOVÁ, Sylva. Otrava železem [online]. ©2003. Poslední revize 2003-10-06, [cit. 2021-08-16]. <http://medicina.cz/clanky/5819/34/Otrava-zelezem/>.
Literature[edit | edit source]
- BUBNOVÁ, Eva, Alena BUDĚŠÍNSKÁ a Jana STŘÍBRNÁ. Praktická cvičení z lékařské chemie a molekulární biologie. 1. vydání. Praha : Karolinum, 1998. 200 s. ISBN 80-7184-725-9.
- BURTIS, Carl A a Edward R ASHWOOD. Tietz textbook of clinical chemistry. 2. vydání. Philadelphia : Saunders, 1994. 2326 s. ISBN 0-7216-4472-4.
- ČERMÁK, J. Současné možnosti léčebného ovlivnění stavů s nedostatkem či nadbytkem železa v organismu. Remedia. 1994, roč. 4, s. 254-259, ISSN 0862-8947.
- DOLEŽALOVÁ, Věra, et al. Laboratorní technika v klinické biochemii a toxikologii. 4. vydání. Brno : Institut pro další vzdělávání pracovníků ve zdravotnictví, 1995. 286 s. ISBN 80-7013-198-5.
- DZÚRIK, Rastislav. Štandardná klinickobiochemická diagnostika. 2. vydání. Martin : Osveta, 1996. 464 s. ISBN 80-217-0256-7.
- CHROMÝ, Vratislav. Analytické metody v klinické chemii. 1. vydání. Brno : Masarykova univerzita, Přírodovědecká fakulta, 2000. 211 s. ISBN 80-210-2363-5.
- KAPLAN, Lawrence A, Amadeo J. PESCE a Steven C. KAZMIERCZAK. Clinical chemistry. 3. vydání. St. Louis : Mosby, 1996. 1064 s. ISBN 0-8151-5243-4.
- Kolektiv autorů. . Lékařská chemie a biochemie. 1. vydání. Praha, Bratislava : Avicenum, Osveta, 1991. 237 s. sv. 2. ISBN 80-201-0114-4.
- KRAML, Jiří. Návody k praktickým cvičením z lékařské chemie a biochemie. 4. vydání. Praha : Karolinum, 1991. 311 s. ISBN 80-7066-453-3.
- MASOPUST, Jaroslav. Klinická biochemie : požadování a hodnocení biochemických vyšetření.. 1. vydání. Praha : Karolinum, 1998.. 832 s. ISBN 80-7184-650-3.
- MEŠKO, Dušan. Vademékum klinickej biochémie. 1. vydání. Martin : Osveta, 1998. 1647 s. ISBN 80-8063-005-4.
- MURRAY, Robert K, et al. Harperova biochemie. 2. vydání. Praha : H & H, 1998. 872 s. ISBN 80-85787-38-5.
- RACEK, Jaroslav, et al. Klinická biochemie. 1. vydání. Praha : Galén, 1999. ISBN 80-7262-023-1.
- SCHNEIDERKA, Petr. Stanovení analytů v klinické biochemii. 1. vydání. Praha : Karolinum, 1999. 153 s. sv. 1. ISBN 80-7184-761-5.
- SCHNEIDERKA, Petr. Vybrané kapitoly z klinické biochemie. 1. vydání. Praha : Karolinum, 1998. 119 s. ISBN 80-7184-505-1.
- ŠTÍPEK, Stanislav. Stručná toxikologie. 1. vydání. Praha : Medprint, 1997. 87 s. ISBN 80-902036-4-7.
- TÁBORSKÁ, Eva a Josef TOMANDL, et al. Biochemie. 1. vydání. Brno : Masarykova univerzita, 1998. 160 s. ISBN 80-210-1736-8.
- TÁBORSKÝ, Otto, et al. Metody klinické biochemie : určeno pro posluchače fakulty přírodovědecké. 2. vydání. Praha : SPN, 1990. 158 s.
- THOMAS, Lothar, et al. Clinical Laboratory Diagnostics. 1. vydání. Frankfurt am Mein : TH-Books Verlagsgeselschaft, 1998. 1727 s. ISBN 3-9805215-4-0.
- ZIMA, Tomáš, et al. Laboratorní diagnostika. 1. vydání. Praha : Galén, 2003. 728 s. ISBN 80-7262-201-3.

![{\displaystyle \mathrm {H} _{2}\mathrm {O} _{2}+\mathrm {H} _{2}\mathrm {A} \ {\xrightarrow[{\mathrm {nebo\ hemoglobin\ a\ jin{\acute {e}}\ l{\acute {a}}tky} }]{\mathrm {peroxid{\acute {a}}zy} }}\ 2\ \mathrm {H} _{2}\mathrm {O} +\mathrm {A} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/23377fd4d265df004d50c7657f43bcbbe4cdc8ff)

![{\displaystyle {\mbox{Saturace transferinu }}[\%]={\frac {{\mbox{S-}}{\check {\mbox{z}}}{\mbox{elezo }}[\mu {\mbox{mol/l}}]}{{\mbox{S-transferin }}[{\mbox{g/l}}]\times 25,2}}\times 100}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8eeba34f5d6381b5048bf7f12089baea1a98b6b3)