Glass Electrode / Details
The glass electrode is an ion-selective electrode (ISE) that is used to measure the activity of hydrogen ions H + , in practice to determine the pH of a solution .
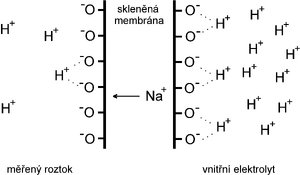
Ions, mainly hydrogen and alkali metals (especially sodium), are bound to the relatively regular silicate crystal lattice of glass by electrostatic forces . When in contact with the solution, a solvated layer is formed on the surface, in which hydrogen ions (but also, for example, sodium) are exchanged between the solution and the glass. The glass electrode therefore differs from most ISEs in that the generation of the potential is not the result of redox processes, but exchange events taking place between the ions in the crystal lattice of the glass and the ions in the solution.
As already mentioned, hydrogen and sodium ions are most significantly involved in the events on the surface of the glass electrode. If we immerse the electrode in a certain solution and wait for equilibrium to be established, the equilibrium state can be described by the relation:
- (* denotes glass phase)
Ks is the so-called selectivity constant , which shows how selectively the electrode reacts to one or the other ion.
From the relationship stated above, it follows that the potential of the indicator electrode will (simplified) be:
(" const " does not exactly correspond to the standard reduction potential, but includes the type and composition of the glass, the method of preparation of the electrode, the quality of the surface and the internal filling of the electrode).
By editing we get
- .
Dependence of electrode potential on pH, measurement errors[edit | edit source]
The potential of the indicating electrode is therefore linearly dependent on the pH of the measured solution. In real conditions, the voltage on the electrode will increase with increasing pH somewhat more slowly than it would correspond to theoretical assumptions. To correct such deviations, pH meters are calibrated (in two or more pH values) and the slope of the calibration line ( slope , electrode sensitivity) is automatically calculated according to it. At the same time, a temperature correction is performed. The ratio of actual to theoretical slope under optimal conditions is around 98%, decreasing with electrode wear and contamination. For values below 95%, the electrode must be cleaned and treated according to the established procedure.
If we know the function of the glass electrode, it is easy to explain some of the measurement errors with it. The alkaline (positive) error is caused by the selectivity of the glass electrode not being ideal. At very low concentrations of H + , i.e. in a strongly alkaline region, the present Na + ions also start to play a role . Depending on the glass used and the quality of its processing, the sodium error manifests itself when the pH of the solution is above 8–14; the measured pH will be lower than the actual pH. It is reported that when working with solutions with a pH of around 10 and with a concentration of Na + 0.1 mol · l −1the measured value can differ from the actual pH by up to 0.4. In a strongly acidic environment (pH below 1–2), on the contrary, protons will saturate the glass surface and the electrode will cease to be sensitive to further lowering of pH, i.e. we will measure a higher result than it should be in reality; in this case we are talking about an acidic (negative) error (pH < 1).
It is also clear that the movement of ions on the membrane is relatively slow, especially in the presence of colloidal substances in the measured solution, which results in a relatively long time for the electrode to stabilize - usually around 15 to 60 s (in acidic solutions, the responses are faster than in alkaline ones).
Other measurement errors can be caused by the properties of the measured solutions and the handling of the electrode. If the electrode dries out, the surface layer of the hydrated gel is damaged and it takes many hours to restore it. Some substances can also bind to the gel and affect its properties ("electrode poisoning"). The consequences of mechanical impurities on the membrane do not need to be discussed.
Links[edit | edit source]
Related articles[edit | edit source]
Category:Biochemistry
Category:Chemistry
Category:Biophysics