Electrode events
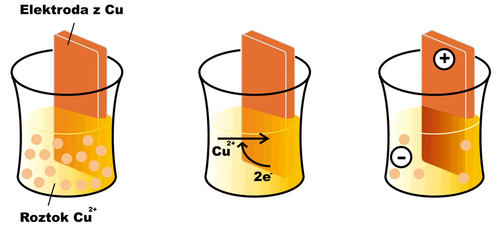
If we dip an electrode made of the same metal (in this case copper) into a solution of a metal ion (e.g. Cu2+) an oxidation-reduction reaction begins to take place on the surface of the electrode
- Cu2+ + 2 e− < = > Cu
In the case of copper, under normal conditions, the equilibrium of the mentioned reaction is shifted somewhat to the right (for other substances, e.g. zinc, the opposite can be the case). A layer of reduced copper is deposited on the surface of the electrode, and the electrode gradually acquires a positive charge, as free electrons are drawn from it. The solution in which the electrode is immersed, on the other hand, acquires a negative charge, as Cu2+ cations are depleted from it without replacement . After a certain time, the reaction stops, as electrostatic forces prevent the further movement of charged particles, and an equilibrium characterized by a certain electric potential at the electrode is established. The effort of the electrode to receive or give up electrons is characterized by the so-called reduction potential (Ered). If all components of the electrode reaction have an activity equal to one, or are in a form to which the standard state is related (e.g. solid state), we speak of a standard reduction potential (E0red).
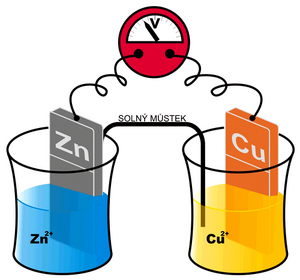
We cannot measure the potential created in the manner described above on one electrode directly. However, we can create a cell composed of two different electrodes – eg metals (generally semi-cells) and two corresponding electrolytes. An example can be the so-called Daniell cell: Cu in a solution of Cu 2+ and Zn in a solution of Zn 2+ . Depending on whether individual metals tend to be more oxidized or reduced, we can arrange them in the so-called Beketov electrochemical series (K, Ca, Al, Zn, Fe, Ni, Pb, H, Bi, Cu, Hg, Ag, Au). For these purposes, the electrode potential of the so-called standard hydrogen electrode is considered ero. Metals that have a negative standard reduction potential and therefore readily donate an electron are in the row to the left of hydrogen (ie. potassium tends to oxidize to K+). Metal ions that are on the right, on the other hand, accept electrons easily (eg. Ag+ is easily reduced to Ag)and their standard reduction potential is positive.
Daniell's article mentioned above is schematically written Zn | Zn2+ || Cu2+ | Cu (the more negative metal is written to the left). A redox reaction takes place at the zinc electrode
- Zn2+ + 2 e− < = > Zn,
which has an equilibrium constant KZn and is characterized by a standard reduction potential E0red(Zn);the above reaction takes place on the copper electrode
- Cu2+ + 2 e− < = > Cu
with the equilibrium KCu and the standard reduction potential E0red(Cu). In the given case, KCu > KZn, or E0red(Cu) > E0red(Zn). In other words, copper will be reduced and deposited on the electrode more readily than zinc. Thus, more electrons are consumed from a copper electrode than from a zinc one. Ultimately, the copper electrode will have a positive voltage to the zinc that we can measure.
If we let a current flow between the electrodes, the „convergig" electrons will be supplied to the copper electrode from the zinc electrode, and the resulting process can be written as follows:
- Zn → Zn2+ + 2e−
- Cu2+ + 2e− → Cu
Current will flow through the conductor between the electrodes and the salt bridge until the zinc electrode dissolves, or (which is more likely) until Cu2+ from the electrolyte is consumed, or the second electrolyte becomes saturated with Zn2+ ions (discharge of the electrochemical cell).
The cell voltage is equal to the potential difference of the two electrodes. A cell on which events take place spontaneously („produces“ voltage) is called a galvanic cell (ΔG < 0). If we apply a voltage to it and the events are "forced" by the applied voltage (ΔG > 0), we call such a cell electrolytic.
The electrode processes described are relatively general and take place in a similar way in various systems composed of various metals and ions.
.