Gastrointestinal stromal tumor / PGS
Gastrointestinal stromal tumor (GIST, sometimes used GISToma form, ICD-O: 8936/0 ) is a mesenchymal tumor of the gastrointestinal wall. Originally mesenchymal tumors that do not show the character of leiomyomas or schwannomas , GISTs now include most gastrointestinal mesenchymal tumors, eg the terms leiomyoma and leiomyosarcoma are intended only for tumors that have convincing histological and immunochemical features of smooth muscle. The ultrastructure of GISTs contains elements of the cells of the autonomic nervous system ( Cajal cells) and smooth muscle, therefore it is assumed that they arise either directly from Cajal cells or from a common precursor of Cajal and smooth muscle cells. Biological behavior can be both benign and malignant, so it is said that GISTs show a spectrum of behavior from benign to malignant. Biological behavior can be predicted mainly by size and mitotic activity. GISTs appear more in old age, the documented difference between the disabilities of individual sexes is not large. Malignant GISTs are not common tumors. For example, GISTs are most common in the stomach, with malignant gastric GIST accounting for approximately 2% of gastric malignancies .
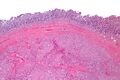
Macroscopic appearance[edit | edit source]
GISTs can be found in any part of the digestive tract, in the omentum and in the mesentery, and case reports describing the occurrence of a tumor with GIST features in other localities are exceptional. The most common sites are:
- stomach: 60-70% of cases
- small intestine: 20-30% of cases
- esophagus and colon: together less than 10% of cases
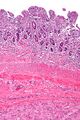
Small GISTs usually present as subserosal, submucosal, or intramural nodules. They are not encapsulated, but they are well demarcated from the surroundings. Large GISTs forgive, their surface is in the lumen is covered by intact mucosa, which, however, can be exulcerated in 20-30% of cases. The appearance of the tumor may be lobed. The tumor can spread directly to surrounding organs, such as the pancreas or liver, and infiltrate their parenchyma. The consistency of the tumor can be stiff or soft, it is usually light brown in cross-section, and intraparenchymal hemorrhages are common. Massive foci of hemorrhagic necrosis and the presence of cystic formations are not exceptional in larger tumors. Multiple occurrence is possible, usually a sign of malignant biological behavior.
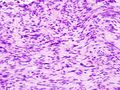
The microscopic appearance[edit | edit source]
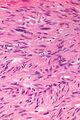
Two extreme extremes in the dominant histological picture of GISTs are described. Most GISTs are spindle cell, a smaller number of which are epithelioid cells. These cells can be arranged in a variety of patterns. For example, in gastric GISTs, eight characteristic patterns can be defined (see gastric GISTs ), in which most GISTs can be included and biological behavior can be inferred from the group. In contrast, for small intestinal GISTs, such a classification is not very useful.
Both spindle and epithelioid cells can be arranged in the following patterns:
- fascular arrangement
- storiform arrangement
- palisade arrangement of cores to a character similar to Schwannom
- alveolar arrangement
- formation of organoid structures
The fibrous matrix of GISTs can hyalinize or change myxoidally.
The following features often appear in GISTs that can be diagnostically useful:
- perinuclear vacuolation of various degrees
- skeinoid fibers - eosinophilic and PAS positive globular masses; if Masson's trichrome is used , they are also highlighted by it.
- nuclear pleomorphism occurs more in epithelioid GISTs, but is usually not very pronounced
- perivascular hyalinization
Striking nuclear pleomorphism is unusual for GISTs.
Immunochemical properties[edit | edit source]
- KIT - Membrane tyrosine kinase, its expression is considered by some authors to be defining for GISTs, although KIT negative GISTs have been described. Strong positivity is common, but the staining pattern may be different. The staining pattern may not be constant throughout the preparation, in some areas there may be a loss of staining. Although KIT is a membrane protein, the whole cytoplasm may be positive.
- PDGFRA - α type of growth factor receptor PDGF . PDGFRA mutations are relatively common, so positivity is relatively sensitive. PDGFRA is also expressed in the intra-abdominal desmoid.
- PKC θ - Protein kinase C θ is a serine / threonine kinase. Sensitivity is relatively high, more in GISTs outside the stomach. It is not expressed in fibromas and leiomyomas, it is expressed only rarely in schwannomas.
- CD 34 - Adhesive protein of hematopoietic as well as neural cells. It is detected in 70–80% of cases, a higher proportion is in GISTs of the stomach, on the contrary, in the small intestine only about half of the tumors are positive. Due to its relatively low sensitivity and specificity, it has limited use in the diagnosis of GISTs.
- DOG-1 - Chloride channel, which is also expressed in KIT negative GISTs, is highly sensitive and specific .
- smooth muscle markers - About a fifth of gastric GISTs and a third of small intestinal GISTs stain smooth muscle actin . The positivity of desmin staining is rather exceptional. The positivity of h-caldesmon is relatively common
- S100 protein - Used more in the differential diagnosis of melanomas and schwanomas . Literature data on GIST positivity range from exceptional positivity to 10% of cases.
- keratins - Sometimes there is a positive reaction with keratin 18, less often with keratin 8. The positivity of other keratins has not been observed.
The recommended procedure for histological examination[edit | edit source]
Sample description[edit | edit source]
Partial biopsy[edit | edit source]
- Macroscopic description
- sample type
- sample dimensions (at least the largest dimension)
- Microscopic description
- tumor dimensions (at least the largest dimension)
- tumor location (if identifiable)
- morphological subtype (spindle cell, epithelioid, mixed, ...)
- mitotic index (if determinable)
- presence of necrosis
- tumor grade (if identifiable)
Resection[edit | edit source]
- Macroscopic description
- resection type
- resection dimensions
- tumor localization
- tumor size (at least the largest size)
- relation to resection margins
- distribution (unifocal vs. multifocal)
- mucosal ulceration
- ingrowth of the peritoneum
- description of the cutting surface (bleeding, necrosis, degenerative changes, ...)
- Microscopic description
- morphological subtype (spindle cell, epithelioid, mixed, ...)
- mitotic index
- presence of necrosis
- tumor grade
- risk of aggressive behavior
- resection margins
- stage according to pTNM
- description of changes associated with therapy in already treated tumors (optional)
Further examination[edit | edit source]
Immunochemical tests[edit | edit source]
If the tumor is not smaller than 1 cm and without detected mitoses, the diagnosis should always be verified histochemically, the following three markers are mandatory: KIT, S100 and desmin. These markers will help distinguish between GIST, schwannoma and leiomyoma / leiomyosarcoma:
| KIT | S100 | desmin | note | |
|---|---|---|---|---|
| leiomyoma / leiomyosarcoma | - | - | + | |
| schwannom - | + | - | ||
| melanoma | + | + | - | 2. histochem examination HMB45, melanA |
If staining on KIT, S100 and desmin is negative, the recommended algorithm is as follows:
- Inflammatory appearance of the tumor? If so, then:
- immunohistochemically ALK +, desmin +/-, systemic symptoms: inflammatory myofibroblastic tumor
- intimate contact of spindle cells with lymphocytes, CD21 +, CD35 +: follicular dendritic cell tumor
- submucosa, presence of eosinophils, cocentric formations, CD34 +, PDGFRA mutations: Vanek 's tumor (inflammatory fibroid polyp)
- Do blood vessels look like an endometrium? If so, then:
- CD10 +, estrogen receptors +, positive gynecological history: endometrial stromal sarcoma
- Cytokeratin staining? If so, then:
- extensive sampling: sarcomatoid carcinoma
- None of the above?
- second reading, KIT / PDGFRA mutation analysis: solitary fibrous tumor
Molecular genetic analysis[edit | edit source]
Molecular biology confirms the diagnosis, the result is predictive of the expected response to therapy. It is especially important for metastatic and high-risk tumors.
- KIT gene exon 11 mutation: best response to imatinib therapy
- KIT gene exon 9 mutation: longer progression-free survival with increased imatinib dose, profit from sumatinib administration
- Asp842Val mutation of exon 18 of the PDGFRA gene: imatinib resistance
Clinical behavior[edit | edit source]
Clinical manifestations depend on the size of the tumor and its location. The smallest tumors are usually completely asymptomatic and are an accidental finding. Clinically manifest tumors are most often the cause of gastrointestinal bleeding of varying intensity or indeterminate manifestations in the abdomen. A less common manifestation is wall obstruction or perforation. Palpable meat is more of an exception.
Metastatic spread[edit | edit source]
Malignant GISTs can establish distant metastases. It most often spreads to the liver and soft tissues in the abdomen. Bone and peripheral soft tissue metastases are less common. Lymph node or lung metastases are very rare.
In aggressively behaving GISTs, possible metastases most often appear 1-2 years after resection of the primary tumor. GIST that does not behave aggressively can also be a source of metastasis. These then appear 5–15 years after resection of the primary deposit.
Prognosis[edit | edit source]
Biological behavior can be determined primarily by tumor size and mitotic activity. GISTs less than 5 cm in diameter are usually benign. Other important prognostic factors are mitotic activity as well as histological type. Locality probably also plays a role in the prognostic factor, eg gastric GIST generally has a better prognosis than GIST of the same type and the same mitotic activity in the small intestine.
| size | mitotic activity¹ | stomach | jejunum a ileum | duodenum | rectum |
|---|---|---|---|---|---|
| under 2 cm | low | none | none | none | none |
| 2–5 cm | low | very small | small | small | small |
| 5–10 cm | low | small | high | high² | high² |
| over 10 cm | low | medium | high | high² | high² |
| under 2 cm | high | none | none | high³ | high |
| 2–5 cm | high | medium | high | high | high |
| 5–10 cm | high | high | high | high² | high² |
| over 10 cm | high | high | high | high² | high² |
- ¹ low mitotic activity here means at most 5 mitoses per 50 fields of view at the highest magnification, ie mitotic index at most 5
- ² small number of cases, statistical value is not reliable
- ³ very few or no cases
- Other prognostic markers that worsen the prognosis are:
- increased Ki67 expression - high proliferative activity
- loss of p16 expression
- multiple occurrence
- infiltrative growth
- presence of necrosis
- ulceration over the tumor The prognostic factors are:
- presence of skeinoid fibers (small intestine only)
- nuclear palisade Of the molecular biological markers, mutational analysis of the KIT and PDGFRA genes appears to be useful .
Grading[edit | edit source]
GIST grading is determined solely by mitotic index. There are only two levels:
- G1 - maximum 5 mitoses / mm²
- G3 - more than 5 mitoses / mm²
Clinical stage[edit | edit source]
Clinical stages according to the 7th edition of the American Joint Committee on Cancer: Cancer Staging Manual (2010) :
| stage | T | N | M | mitotic index |
|---|---|---|---|---|
| stage IA | T1 or T2 | N0 | M | low |
| stage IB | T3 | N0 | M0 | low |
| stage II | T1 | N0 | M0 | high |
| stage II | T2 | N0 | M0 | high |
| stage II | T4 | N0 | M0 | low |
| stage IIIA | T3 | N0 | M0 | high |
| stage IIIB | T4 | N0 | M0 | high |
| stage IV | any T | N1 | M0 | arbitrary |
| stage IV | any T | any N | M1 | arbitrary |
| stage | T | N | M | mitotic index |
|---|---|---|---|---|
| stage I | T1 or T2 | N0 | M0 | low |
| stage II | T3 | N0 | M0 | low |
| stage IIIA | T1 | N0 | M0 | high |
| stage IIIA | T4 | N0 | M0 | low |
| stage IIIB | T2 | N0 | M0 | high |
| stage IIIB | T3 | N0 | M0 | high |
| stage IIIB | T4 | N0 | M0 | high |
| stage IV | any T | N1 | M0 | arbitrary |
| stage IV | any T | any N | M1 | arbitrary |
Behavior in different organs[edit | edit source]
GIST esophagus[edit | edit source]
GISTs are rare in the esophagus, accounting for only about 1-2% of all GISTs. They are 3 times less common than leiomyomas . They are more common in the lower third of the esophagus in elderly patients. Larger GISTs may be clinically manifested by dysphagia, but growth into the mediastinum without swallowing disorders has also been reported. The histological picture is more often the spindle cell. Few cases have been described to assess the long-term prognosis, but the prognosis seems rather worse.
GIST of the stomach[edit | edit source]
Gastric GISTs represent about 60% of all GISTs. They occur at an older age, only about 10% of cases occur in patients under 40 years of age. About a fifth to a quarter of all gastric GISTs behave malignantly.
The described size of GIST varies from a few millimeters to more than 40 cm, most often around 6 cm. Malignant GISTs may be associated with surrounding organs.
8 histological types of gastric GIST can be defined, but only 70% of tumors can be classified into them:
- sclerosing spindle cell GIST - usually small, with low mitotic activity, abundant collagen trees, sometimes calcified.
- palisade-vacuolated spindle cell GIST - palisade of nuclei resembling Schwannoma structures is evident, another characteristic feature is the perinuclear vacuole. These are usually larger tumors with low mitotic activity.
- hypercellular spindle cell GIST - tight aggregation of uniform spindle cells without distinct palisade of nuclei. Mitotic activity is usually not pronounced, but is sometimes slightly higher.
- sarcomatous spindle cell GIST - significant mitotic activity is evident, diffuse atypia manifested mainly by nuclear hyperchromasia, their enlargement and possibly nuclear pleomorphism. Sometimes a myxoid tree is formed in which streaks of tumor cells can be observed.
- sclerosing epithelioid GIST - tumor cells are polygonal, appear as syncytia, cell boundaries are not visible, the stroma is sclerosing. Nuclear atypia can be observed quite commonly. Mitotic activity is rather low.
- discohesive epithelioid GIST - epithelioid cells are sharply demarcated, a lacunar clearing is visible around the cells. Nuclear pleomorphism is common.
- hypercellular epithelioid GIST - cells have clearly visible boundaries, they are tightly crowded, mitotic activity is low.
- sarcomatous epithelioid GIST - cells have epithelioid morphology, their mitotic activity is significant.
In addition to size and mitotic activity, the histological type is also an important prognostic marker. Roughly (for details, see Miettinen and Lasota, 2006), the risk of metastatic spread is as follows:
- low risk: sclerosing spindle cell GIST, palisade-vacuolated spindle cell GIST and sclerosing epithelioid GIST
- medium risk: hypercellular spindle cell GIST, discohesive epithelioid GIST and hypercellular epithelioid GIST
- high risk: sarcomatous spindle cell GIST, sarcomatous epithelioid GIST
Duodenal GIST[edit | edit source]
About 4-5% of all GISTs are present in the duodenum. The tumor can expand into the pancreas and thus clinically and macroscopically mimic the tumor of this site. Spindle cell tumors are more common, about half contain skeinoid fibers (globular PAS positive masses). Extensive vascularization occurs in about one-fifth of cases, so the tumor resembles a hemangioma. The prognosis of small tumors with low mitotic activity is excellent, while tumors larger than 5 cm in diameter or with high mitotic activity are characterized by high mortality.
GIST intestine[edit | edit source]
About 30% of all GISTs occur in the jejunum and ileum, but they represent a higher proportion of malignant GISTs. The overall mortality is 40-50%. They are more common in elderly patients with a slight predominance of men. Unlike gastric GISTs, small intestinal GISTs do not form clearly distinguishable histological types. The cells are most often spindle-shaped, they can also be epithelioid. Sarcomatous appearance is relatively rare.
GIST large intestine, appendix[edit | edit source]
Colon and appendix GISTs represent about 1-2% of all GISTs. They are more common in old age, more common on the right. Invasion of the muscularis propria is mentioned in the literature as a prognostic marker, but other authors question the significance. The relatively frequent (up to 25% of cases) KIT negativity is also mentioned; it is added that these are probably not GISTs but true sarcomas .
Rectal GIST[edit | edit source]
RIST GISTs represent about 4% of all GISTs. They include small incidentalomas and large sarcomatous tumors. When growing into the prostate, they may appear to be the primary tumor of the prostate. The histological structure of rectal GISTs often contains hyalinized calcifying stroma or palisade arrangements, but only with a faint perinuclear vacuole. Malignant GISTs may have a fascicular structure similar to leiomyosarcoma. The epithelioid variant is rare here. Skeinoid fibers do not usually occur. They may appear clinically indifferent unless they cause obstruction or bleeding.
GIST outside GIT[edit | edit source]
Outside the GIT, more precisely outside the gastrointestinal tract, primary GISTs are relatively rare. They most often occur in the omentum, mesentery and retroperitoneum. Only a few case reports describe GIST of the gallbladder, larynx and bladder.
Because metastatic spread is much more likely than the primary formation of GIST outside the gastrointestinal tract, it is discussed to what extent, especially in the case of extremely unusual sites, it is indeed a primary tumor and to what extent it is a metastasis from an overlooked primary site.
Differential diagnosis[edit | edit source]
KIT negative GIST[edit | edit source]
Immunohistochemical KIT negativity may occur in 2% of small intestinal GISTs and in 5-10% of gastric GISTs. For small biopsy specimens, negativity is more common because there is a higher risk that only a histochemically non-staining area will be included in the biopsy. In this case, the diagnosis is based on histological structure, it can be confirmed by mutation analysis KIT and PDGFRA.
Leiomyoma[edit | edit source]
Leiomyomas occur less frequently, the ratio to GISTs is 3: 1, in the stomach and small intestine their frequency is even lower. Leiomyomas are usually less cellular, their cells are well differentiated, eosinophilic, immunohistochemically stained for actin and myosin. Mast cells that express KIT on their surface can be infiltrated .
Intestinal wall leiomyomas usually present as small sessile polyps. In women, uterine-type leiomyomas may occur in the abdominal cavity, with no apparent connection to the gut. In addition, they express estrogen and progesterone receptors.
Leiomyomatosis peritonealis disseminata consists of fibroids with low mitotic activity, good differentiation and immunochemical properties of uterine fibroids.
Leiomyosarcoma[edit | edit source]
Primary leiomyosarcoma is rare in the gastrointestinal tract, more often occurring in the large intestine. Leiomyosarcomas are composed of well-differentiated smooth muscle cells with a typical cigarette nucleus. Retroperitoneal leiomyosarcomas are mostly spindle cell, while retroperitoneal GISTs are more composed of epithelioid cells. Immunohistochemically, the actin is positive , usually desmin . KIT is usually negative, only occasionally focal cytoplasmic positivity can be detected.
Glomus tumor[edit | edit source]
Glomus tumor (glomangioma) is an uncommon benign tumor arising from arteriovenous shunts, glomus bodies. It is usually peripheral, occurring in the gastrointestinal tract is not common. Epithelioid GISTs may occasionally be arranged in nests, sometimes arranged around prominent dilated vessels. This may resemble the histology of the glomus tumor.
Glomus tumor is immunochemically positive for smooth muscle actin staining, collagen IV and laminin are detected pericellularly . In contrast, KIT , desmin and S100 protein are not detectable. In about a third of cases, the CD is provable .
Intra-abdominal desmoid[edit | edit source]
Intra-abdominal desmoid (mesenteric fibromatosis) is a tumor-like fibromatic process that is more common in young and middle-aged adults. Histologically, it consists of spindle to stellate fibroblasts scattered in an abundant collagen tree with slightly dilated vessels. Desmoid does not express KIT , previously reported positivity is now considered more of a false positivity of poor quality antibodies. CD 34 is negative. Focal positivity may occur when staining for actin and desmin. Detection of nuclear β-catenin can be performed in most desmoids, cytoplasmic positivity can also occur in GISTs.
Inflammatory fibroid polyp[edit | edit source]
Inflammatory fibroid polyp is a submucosal fibroblastic inflammatory lesion, most commonly occurring as an ulcerated small intestinal polyp, rather in younger ones. The histological picture can be from a relatively wide range of changes, it can also mimic GIST. Sometimes CD34 can be demonstrated, KIT can never be demonstrated .
Sclerosing mesenteritida[edit | edit source]
Sclerosing mesenteritis is a non-cancerous condition affecting the mesenterium of the small intestine. Relatively pronounced inflammatory infiltration and KIT negativity can be used to differentiate .
Solitary fibrous tumor, hemangiopericytoma[edit | edit source]
Solitary fibrous tumor and hemangiopericytoma have a number of common morphological features, and are often considered to be variants of the same clinical entity. They are almost always positive for CD 34 and negative for actin and desmin. Presence mast cells tend to be KIT positive, mostly perivascular.
Angiosarcoma[edit | edit source]
Angiosarcoma is rare in the digestive tract. Angiosarcoma can be epithelioid or spindle cell, its cells form real vascular structures. Common expression of KIT , for differentiation from GIST must use angiosarcoma markers such as CD 31 and von Willebrand factor (pathological in the literature is known as Factor VIII related antigen ).
Synovial sarcoma[edit | edit source]
Synovial sarcoma can rarely occur in the esophagus, stomach, abdominal wall and retroperitoneum. KIT may not be positive, some studies describe biphasic KIT positivity, ie weak spindle cell positivity and strong epithelial component positivity.
Undifferentiated sarcoma[edit | edit source]
Undifferentiated sarcoma may be macroscopically similar to GIST, but histology shows much greater pleomorphism and much higher mitotic activity than is common in GISTs. KIT and CD 34 tend to be negative.
Dedifferentiated liposarcoma[edit | edit source]
Dedifferentiated liposarcoma may resemble GIST clinically and macroscopically. If lipomatous foci are not present, more pronounced pleomorphism and more visible fibrous trees may be used to differentiate from GIST. Sometimes KIT staining may be present in individual cells .
Gastrointestinal schwannoma[edit | edit source]
Schwannomas are rare in the digestive tract, if they do appear, then more in the stomach or colon. These are usually small spindle cell tumors with low mitotic activity. They are usually surrounded by lymphatic tissue, in which germinal centers can also appear. They tend to be positive for S100 protein and GFAP , and never stain for KIT and CD34.
Gangliocytic paraganglioma[edit | edit source]
Gangliocytic paraganglioma is generally a benign rare tumor, most often occurring in the duodenum around the ampoule. It consists of carcionoid-like elements that are surrounded by Schwannom-like cells mixed with ganglion cells. Unfortunately, even duodenal GISTs tend to have a histologically nesting appearance. They tend to be positive for neuronal tissue markers, and can often be positive for KIT .
Metastatic melanoma[edit | edit source]
Amelanotic malignant melanoma can metastasize to any part of the colon. Due to the richness of histological patterns, it may raise suspicion of GIST. However, melanoma is almost always positive in staining for S100 . Melanomas are also expressed by KIT, but metastases in particular tend to express this protein less.
Perivascular tumor cells of epithelioid[edit | edit source]
The perivascular tumor from epithelioid cells is a tumor from the group of angiomyolipomas. It expresses melanocytic markers HMB45, melan A and MITF, usually does not express S100 and only sometimes expresses smooth muscle markers. Usually the KIT is negative.
Histiocytic sarcoma[edit | edit source]
Histiocytic sarcoma is a rare aggressive tumor. He may have differential diagnostic problems in relation to pleomorphic GISTs. It expresses CD 163, CD6], lysozyme and often S100 . Expression of epithelial markers, lymphatic cells, dendritic cells and melanocytes is not demonstrable.
Extramedullary myeloid tumor[edit | edit source]
Extramedullary myeloid tumor (myeloid sarcoma, chlorine) is a lesion formed by primitive myeloid cells. It often accompanies some form of leukemia . It is positive for staining for KIT and CD 34, but also expressed markers of the myeloid lineage such as LCA, CD43, myeloperoxidase and lysozyme.
Mastocytoma[edit | edit source]
Mastocytoma can exceptionally manifest as a solitary tumor mass without other characteristic accompanying manifestations. KIT staining is usually positive on cell membranes. It also expresses chloroacetate esterase, tryptase, CD45, CD68 and CD33.
Seminoma and ovarian dysgerminoma[edit | edit source]
Seminoma and ovarian dysgerminoma can be a source of diagnostic embarrassment if they occur in the retroperitoneal localization. In addition to the very different histologies, their positivity for PLAP and OCT4 may be a diagnostic guide.
Metastasis of carcinoma[edit | edit source]
Metastases of some cancers can also be a source of diagnostic embarrassment, especially due to the frequent positivity in KIT staining.
Therapy[edit | edit source]
The basic therapeutic procedure is tumor resection with evidence of intact resection margins. Because GISTs rarely metastasize to the lymph nodes, their biopsy or removal is irrelevant.
GISTs are resistant to conventional chemotherapy, and trials with doxorubicin and ifosfamide, the cytostatics used in sarcoma therapy, have failed. Similarly, GISTs are resistant to radiotherapy.
A key event in the pathogenesis of approximately 80% of GISTs is a mutation in KIT , a transmembrane tyrosine kinase that serves as a receptor for the cytokine SCF (Stem Cell Factor). Inhibition of this kinase by the tyrosine kinase inhibitor imatinib represents a non-surgical approach that has a certain therapeutic effect. The first patient with metastatic and surgically unsolvable GIST was treated in Finland at the turn of the century with marked remission of the disease, including a demonstrable remission of existing metastases. However, a small proportion of patients treated with imatinib experience further disease progression during therapy or growth arrest, but most respond to a greater or lesser degree of resolution, although complete resolution is not common. Imatinib is used in the treatment of unresectable and metastatic GISTs.
Molecular Pathology[edit | edit source]
There is not a single key mutation in the pathogenesis of GISTs. The most common is a mutation in KIT , the receptor for the cytokine SCF (Stem Cell Factor). Mutation in this gene is detectable in about 80% of cases. The second most common mutation is the PDGFRA mutation , the alpha receptor for the cytokine PDGF (Plated Derived Growth Factor). This mutation is detectable in about 10% of cases. Because they are both receptors with tyrosine kinase activity, GISTs are sensitive to imatinib in both cases . Both receptors are from family III receptors with tyrosine kinase activity, they further transmit the signal by phosphorylation of proteins from the MAPK, PI3K and p90RSK signaling cascades. In both cases, the mutation leads to a permanent activation of the receptor.
Germ cell mutation syndromes with KIT and PDGFRA have been described. GIST penetration is practically 100% for these syndromes. Cajal cell hyperplasia is common. KIT mutations further manifest as urticaria pigmentosa and cutaneous hyperpigmentation.
The remaining about 10% of tumors do not have a mutation in any of the above genes. Tumors without mutations in the KIT and PDGFRA genes are referred to as the wild-type phenotype.
KIT mutations in GIST[edit | edit source]
The KIT mutation was described in GIST in 1998. KIT is a transmembrane receptor that crosses the cell membrane once. Ligand binding (SCF) allows for close approximation of the two monomers, their binding and autophosphorylation of the intracellular domains. The autophosphorylation-activated receptor further activates the MAPK, PI3K and p90RSK signaling cascade proteins.
The distribution of mutations is not uniform along the entire gene:
- mutations in exon 11: 65–70% of cases (ie all GISTs)
- mutations in exon 9: 10-15% of cases
- mutations in exon 13: 1.2% of cases
- mutations in exon 17: 0.6% of cases
- mutations in exon 8: 0.2% of cases (only a few case reports)
Exon 11 encodes a receptor region located close to the cell membrane from the intracellular side. Mutations in exon 11 allow the receptor to assume a conformation leading to activation even without ligand binding. Deletions, insertions and substitutions occur, deletions are associated with a worse prognosis compared to other mutations in exon 11.
Exon 9 encodes a region adjacent to the cell membrane from the extracellular side. Biologically, it is a region that promotes receptor dimerization, which is one of the steps in activation. The exon 9 mutation is associated with lower GIST sensitivity to imatinib. Mutations in exon 9 are more common in small and large intestinal GISTs and are less common in gastric disorders.
Exon 13 encodes the ATP binding region of the tyrosine kinase domain. Exon 17 encodes an activating loop of the tyrosine kinase domain, its oncogenic mutations leading to greater stability of the activated receptor. These two mutations are more common in small intestinal GISTs. Interestingly, their histopathological correlate is usually the spindle cell morphology of the tumor.
The marked and diffuse cytoplasmic immunoreactivity at KIT is characteristic of GISTs with a KIT mutation, regardless of which part of the gene is affected.
GISTs with PDGFRA mutations[edit | edit source]
The PDGFRA mutation was described in GISTs without the KIT mutation in 2003. The structure and function of the receptor is similar to the KIT receptor.
The distribution of mutations is not uniform along the entire gene:
- mutations in exon 18: 6% of cases
- mutations in exon 12: 1.5% of cases
- mutations in exon 14: 2% of cases
Exon 18 encodes a tyrosine kinase domain activation loop. Exon 12 encodes a domain adjacent to the inside of the cell membrane. Exon 14 encodes the ATP binding region of the tyrosine kinase domain.
Additional genetic changes[edit | edit source]
Other characteristic changes in genetic material often develop in GISTs. Chromosome 14 is affected in about two-thirds of the cases, with chromosome 14 monosomy and 14q deletion being the most common. In about half of the cases, a deletion of 22q is evident. Insertions at 8q, 3q and 17 are common, and are associated with more aggressive tumor behavior.
Associations with aggressive behavior are also likely in mutations in other genes, notably CDKN2A (cyclin dependent kinase 2A inhibitor), the P53 T gene, and the PI3K cascade.
GIST without KIT mutations and PDGFRA[edit | edit source]
This group represents 10-15% of adult GISTs, but for children they represent up to 90% of all GISTs. Clinically, it is a heterogeneous group of tumors.
GIST deficient SDH[edit | edit source]
Succinyl dehydrogenase is actually complex II of the respiratory chain . Loss of activity of units A (SDHA) and B (SDHB) is a characteristic feature of GISTs formed as part of the Carney Triassic (non-hereditary occurrence of GISTs of the stomach, paragangliomas and pulmonary chondromas ) and Carney's Stratakis syndrome (hereditary occurrence of GISTs of the stomach and paragangliomas). Because they are usually (though not exclusively) childhood tumors, SDH deficient GISTs are sometimes referred to as pediatric GISTs.
They occur more often in women, almost exclusively in the stomach. Unlike other GISTs, lymph node metastases occur here. SDH deficient GISTs tend to be resistant to imatinib. The development of the disease is gradual, cases of patients who have survived for decades with proven metastases in the liver or peritoneum are described.
Although KIT's characteristic positivity is demonstrated by staining, the gene itself is not mutated. Loss of expression of the SDHB and SDHA subunits is demonstrated immunochemically. While loss of SDHA is associated exclusively with gene mutation, loss of SHDB expression is a manifestation of a wider range of disorders involving the entire respiratory chain complex II.
The mechanism by which loss of the succinate dehydrogenase complex leads to tumor formation has not been fully elucidated. However, the mechanism appears to be the accumulation of succinate. The increase in succinate induces the stabilization of the transcription factor HIF1-α]], which then leads to the transcription of, among others, the gene for the cytokine VEGF (Vascular Endothelial Growth Factor).
The accumulation of succinate also inhibits some enzymes dependent on the presence of α-ketoglutarate, especially DNA hydroxylase from the TET family, thereby disrupting, inter alia, 5-hydroxymethylcytosine production. Because DNA methylation has been shown to be lower in SDH-deficient GISTs than in GISTs caused by KIT mutations, it appears that the altered DNA methylation pattern may play a role in pathogenesis.
Overexpression of the IGF1 receptor gene (Insulin-like Growth Factor 1) is a relatively common finding in this group of GISTs. The mechanism and significance are still unclear.
GISTs are associated with neurofibromatosis type I.[edit | edit source]
The pathogenesis of GISTs in patients with type I neurofibromatosis is unknown, although an association is evident. GISTs most often occur in the small intestine, neither the KIT mutation nor the PDGFRA mutation is detectable. GISTs often arise from Cajal cell hyperplasia. An immunochemical peculiarity is that approximately one third of GISTs associated with neurofibromatosis show evidence of S100 protein. Even in these GISTs, the characteristic staining on the KIT is immunochemically evident. The behavior of these GISTs is usually biologically favorable.
GIST BRAF mutation[edit | edit source]
Mutations in exon 15 of the BRAF gene were identified in approximately 13% of wild-type GISTs. B-Raf is a serine / threonine kinase playing an important role in the RAS-RAF-ERK signaling cascade.
These GISTs occur most often in the small intestine, less often in the stomach. Probably (few data) these are highly malignant tumors that are resistant to imatinib, but show some sensitivity to the BRAF inhibitor dabrafenib .
Image gallery[edit | edit source]
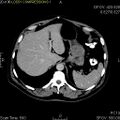
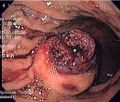
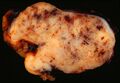
- Macroscopic appearance
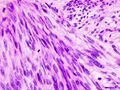
- Microscopic appearance
- Immunochemical staining
References[edit | edit source]
Related Articles[edit | edit source]
- Risk of progression and staging of GISTs (table, PDF, one page A4)
- Mesenchymal tumors
- Stomach tumors
References[edit | edit source]
- ROSAI, Juan. Ackerman's Surgical Pathology. 8th edition. St. Louis, MO: Mosby, 1996. vol. 1. ISBN 0-8016-7004-7 .
- HAMILTON, Stanley R. and Lauri A. AALTONEN. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Digestive System, [online] . 1st edition. Lyon: IARC Press, 2000. Also available from < http://publications.iarc.fr >. ISBN 92-832-2410-8 .
- WHO. . International Classification of Diseases for Oncology: Czech version. 3rd edition. Prague: Institute of Health Information and Statistics, 2004. ISBN 80-7280-373-5 .
- LASOTA, J, JA CARLSON and M MIETTINEN. Spindle cell tumor of urinary bladder serosa with phenotypic and genotypic features of gastrointestinal stromal tumor. Arch Path Lab Med [online] . 2000, vol. 124, vol. 6, pp. 894-897, also available from < http://www.archivesofpathology.org/doi/full/10.1043/0003-9985%282000%29124%3C0894:SCTOUB%3E2.0.CO; 2 >. ISSN 1543-2165.
- MIETTINEN, M and J LASOTA. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. No Diagn Pathol. 2006, vol. 23, vol. 2, pp. 70-83, ISSN 0740-2570.
- DOW, N and G GIBLEN, et al. Gastrointestinal stromal tumors: Differential diagnosis. No Diagn Pathol. 2006, vol. 23, vol. 2, pp. 111-119, ISSN 0740-2570.
- KOH, JS, J TRENT and L CHEN, et al. Gastrointestinal stromal tumors: Overview of pathologic features, molecular biology, and therapy with imatinib mesylate. Histol Histopatol. 2004, vol. 19, vol. 2, pp. 565-574, ISSN 1699-5848.
- DOYLE, LA and JL HORNICK. Gastrointestinal stromal tumors: From KIT to succinate dehydrogenase. Histopathology. 2014, vol. 64, vol. 1, pp. 53-67, ISSN 1365-2559.
- National Cancer Institute (USA). Gastrointestinal Stromal Tumors Treatment: Stage Information for Gastrointestinal Stromal Tumors [online]. [feeling. 4/2014]. < https://www.cancer.gov/types/soft-tissue-sarcoma/hp/gist-treatment-pdq#section/all >.
- DAUM, O. and M. ŠEDIVCOVÁ. Recommended procedure for histological examination of gastrointestinal stromal tumors. Society of Czech Pathologists, 2014.
External links[edit | edit source]
- reGISTer - registry for the collection of epidemiological and clinical data of patients with gastrointestinal stromal tumor
- BEHAZIN, Nancy S .. Medscape: Gastrointestinal Stromal Tumor [online]. © 2013. [feeling. 2014]. < https://emedicine.medscape.com/article/278845-overview ,>.
- CHOTI, Michael. Medscape: Gastric Gastrointestinal Stromal Tumor [online]. © 2013. [feeling. 2014]. < https://emedicine.medscape.com/article/278845-overview >.
- PathologyOutlines.com. Stomach> Stromal / other tumors> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/stomachGIST.html >.
- PathologyOutlines.com. Esophagus> Other malignancies> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/esophagusGIST.html >.
- PathologyOutlines.com. Small bowel (small intestine)> Other malignancies> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/smallbowelGIST.html >.
- PathologyOutlines.com. Colon tumor> Mesenchymal tumors> Gastrointestinal stromal tumors (GIST) of colon [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/colontumorgist.html >.
- PathologyOutlines.com. Appendix> Other tumors> Gastrointestinal stromal tumors (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/appendixGIST.html >.
- PathologyOutlines.com. Liver and intrahepatic bile ducts - tumor> Other malignancies> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/livertumorGIST.html >.
- PathologyOutlines.com. Stains> DOG-1 [online]. © 2011. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/stainsDOG1.html >.
- ŽABKA, J. Gastrointestinal stromal tumors. Klin Cnkol [online] . 2011, vol. 24, vol. 3, pp. 187-194, also available from < http://www.eonkologie.cz/klinicka-onkologie/archiv/2011/53-archiv/2011-3/248-2011-03zabka >. ISSN 0862-495X.
- DAUM, O, R ŠÍMA and M MICHAL. Pathological diagnosis of gastrointestinal stromal tumor. Oncology [online] . 2010, vol. 4, vol. 1, pp. 13-17, also available from < http://www.onkologiecs.cz/pdfs/xon/2010/01/04.pdf >. ISSN 1803-5345.