Gastrointestinal stromal tumor
Gastrointestinal stromal tumor (GIST, sometimes used GISToma form, ICD-O: 8936/0 ) is a mesenchymal tumor of the gastrointestinal wall. Originally mesenchymal tumors that do not show the character of leiomyomas or schwannomas , GISTs now include most gastrointestinal mesenchymal tumors, eg the terms leiomyoma and leiomyosarcoma are intended only for tumors that have convincing histological and immunochemical features of smooth muscle. The ultrastructure of GISTs contains elements of the cells of the autonomic nervous system ( Cajal cells) and smooth muscle, therefore it is assumed that they arise either directly from Cajal cells or from a common precursor of Cajal and smooth muscle cells. Biological behavior can be both benign and malignant, so it is said that GISTs show a spectrum of behavior from benign to malignant. Biological behavior can be predicted mainly by size and mitotic activity. GISTs appear more in old age, the documented difference between the disabilities of individual sexes is not large. Malignant GISTs are not common tumors. For example, GISTs are most common in the stomach, with malignant gastric GIST accounting for approximately 2% of gastric malignancies .
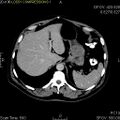
Macroscopic appearance[edit | edit source]
GISTs can be found in any part of the digestive tract, in the omentum and in the mesentery, and case reports describing the occurrence of a tumor with GIST features in other localities are exceptional. The most common sites are:
- stomach: 60-70% of cases
- small intestine: 20-30% of cases
- esophagus and colon: together less than 10% of cases
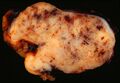
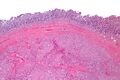
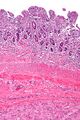
Small GISTs usually present as subserosal, submucosal, or intramural nodules. They are not encapsulated, but they are well demarcated from the surroundings. Large GISTs forgive, their surface is in the lumen is covered by intact mucosa, which, however, can be exulcerated in 20-30% of cases. The appearance of the tumor may be lobed. The tumor can spread directly to surrounding organs, such as the pancreas or liver, and infiltrate their parenchyma. The consistency of the tumor can be stiff or soft, it is usually light brown in cross-section, and intraparenchymal hemorrhages are common. Massive foci of hemorrhagic necrosis and the presence of cystic formations are not exceptional in larger tumors. Multiple occurrence is possible, usually a sign of malignant biological behavior.
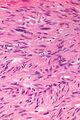
The microscopic appearance[edit | edit source]
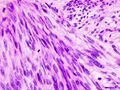
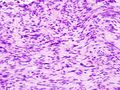
Two extreme extremes in the dominant histological picture of GISTs are described. Most GISTs are spindle cell, a smaller number of which are epithelioid cells. These cells can be arranged in a variety of patterns. For example, in gastric GISTs, eight characteristic patterns can be defined (see gastric GISTs ), in which most GISTs can be included and biological behavior can be inferred from the group. In contrast, for small intestinal GISTs, such a classification is not very useful.
The fibrous matrix of GISTs can hyalinize or change myxoidally. Other relatively characteristic changes may occur in tumors, especially the presence of skeinoid fibers , which are eosinophilic PAS positive globular masses. Striking nuclear pleomorphism is unusual for GISTs.
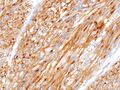
Of the immunochemical properties, the identification of KIT and possibly PDGFRA is especially important . Molecular analysis of mutations in these genes is also important, as prognosis and sensitivity to therapy may vary from mutation to mutation.
Clinical behavior[edit | edit source]
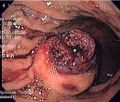
Clinical manifestations depend on the size of the tumor and its location. The smallest tumors are usually completely asymptomatic and are an accidental finding. Clinically manifest tumors are most often the cause of gastrointestinal bleeding of varying intensity or indeterminate manifestations in the abdomen. A less common manifestation is wall obstruction or perforation. Palpable meat is more of an exception.
Metastatic spread[edit | edit source]
Malignant GISTs can establish distant metastases. It most often spreads to the liver and soft tissues in the abdomen. Bone and peripheral soft tissue metastases are less common. Lymph node or lung metastases are very rare.
In aggressively behaving GISTs, possible metastases most often appear 1-2 years after resection of the primary tumor. GIST that does not behave aggressively can also be a source of metastasis. These then appear 5–15 years after resection of the primary deposit.
Prognosis[edit | edit source]
Biological behavior can be determined primarily by tumor size and mitotic activity. GISTs less than 5 cm in diameter are usually benign. Other important prognostic factors are mitotic activity as well as histological type. Locality probably also plays a role in the prognostic factor, eg gastric GIST generally has a better prognosis than GIST of the same type and the same mitotic activity in the small intestine.
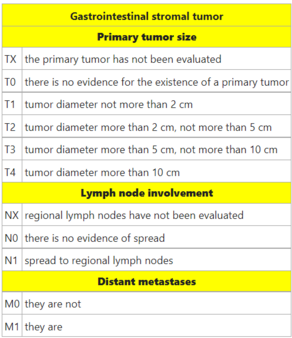
TNM staging[edit | edit source]
Therapy[edit | edit source]
The basic therapeutic procedure is tumor resection with evidence of intact resection margins. Because GISTs rarely metastasize to the lymph nodes, their biopsy or removal is irrelevant.
GISTs are resistant to both conventional chemotherapy and radiotherapy. Due to the molecular nature of most GISTs, some success can be achieved with the tyrosine kinase inhibitor imatinib .
Molecular Pathology[edit | edit source]
There is not a single key mutation in the pathogenesis of GISTs. The most common is a mutation in KIT , the receptor for the cytokine SCF (Stem Cell Factor). Mutation in this gene is detectable in about 80% of cases. The second most common mutation is the PDGFRA mutation , the alpha receptor for the cytokine PDGF (Plated Derived Growth Factor). This mutation is detectable in about 10% of cases. Because they are both receptors with tyrosine kinase activity, GISTs are sensitive to imatinib in both cases . Both receptors are from family III receptors with tyrosine kinase activity, they further transmit the signal by phosphorylation of proteins from the MAPK, PI3K and p90RSK signaling cascades. In both cases, the mutation leads to a permanent activation of the receptor.
Germ cell mutation syndromes with KIT and PDGFRA have been described. GIST penetration is practically 100% for these syndromes. Cajal cell hyperplasia is common. KIT mutations further manifest as urticaria pigmentosa and cutaneous hyperpigmentation.
The remaining about 10% of tumors do not have a mutation in any of the above genes. Tumors without mutations in the KIT and PDGFRA genes are referred to as the wild-type phenotype.
KIT mutations in GIST[edit | edit source]
The KIT mutation was described in GIST in 1998. KIT is a transmembrane receptor that crosses the cell membrane once. Ligand binding (SCF) allows for close approximation of the two monomers, their binding and autophosphorylation of the intracellular domains. The autophosphorylation-activated receptor further activates the MAPK, PI3K and p90RSK signaling cascade proteins.
The distribution of mutations is not uniform along the entire gene, most often mutations occur in exon 11 and exon 9. Significant and diffuse cytoplasmic immunoreactivity at KIT is characteristic of GISTs with KIT mutation, regardless of which part of the gene is affected.
GISTs with PDGFRA mutations[edit | edit source]
The PDGFRA mutation was described in GISTs without the KIT mutation in 2003. The structure and function of the receptor is similar to the KIT receptor. The distribution of mutations is not uniform along the entire gene, mutations in exons 18, 14 and 12 are usually detected.
GIST without KIT mutations and PDGFRA[edit | edit source]
This group represents 10-15% of adult GISTs, but for children they represent up to 90% of all GISTs. Clinically, it is a heterogeneous group of tumors.
- Succynyl dehydrogenase deficient GISTs
- GISTs associated with type I neurofibromatosis
- GISTs with BRAF mutation
Image gallery[edit | edit source]
- Macroscopic appearance
- Microscopic appearance
- Immunochemical staining
References[edit | edit source]
Related Articles[edit | edit source]
- Risk of progression and staging of GISTs (table, PDF, one page A4)
- Mesenchymal tumors
- Stomach tumors
References[edit | edit source]
- ROSAI, Juan. Ackerman's Surgical Pathology. 8th edition. St. Louis, MO: Mosby, 1996. vol. 1. ISBN 0-8016-7004-7 .
- HAMILTON, Stanley R. and Lauri A. AALTONEN. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Digestive System, [online] . 1st edition. Lyon: IARC Press, 2000. Also available from < http://publications.iarc.fr >. ISBN 92-832-2410-8 .
- WHO. . International Classification of Diseases for Oncology: Czech version. 3rd edition. Prague: Institute of Health Information and Statistics, 2004. ISBN 80-7280-373-5 .
- LASOTA, J, JA CARLSON and M MIETTINEN. Spindle cell tumor of urinary bladder serosa with phenotypic and genotypic features of gastrointestinal stromal tumor. Arch Path Lab Med [online] . 2000, vol. 124, vol. 6, pp. 894-897, also available from < http://www.archivesofpathology.org/doi/full/10.1043/0003-9985%282000%29124%3C0894:SCTOUB%3E2.0.CO; 2 >. ISSN 1543-2165.
- MIETTINEN, M and J LASOTA. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. No Diagn Pathol. 2006, vol. 23, vol. 2, pp. 70-83, ISSN 0740-2570.
- DOW, N and G GIBLEN, et al. Gastrointestinal stromal tumors: Differential diagnosis. No Diagn Pathol. 2006, vol. 23, vol. 2, pp. 111-119, ISSN 0740-2570.
- KOH, JS, J TRENT and L CHEN, et al. Gastrointestinal stromal tumors: Overview of pathologic features, molecular biology, and therapy with imatinib mesylate. Histol Histopatol. 2004, vol. 19, vol. 2, pp. 565-574, ISSN 1699-5848.
- DOYLE, LA and JL HORNICK. Gastrointestinal stromal tumors: From KIT to succinate dehydrogenase. Histopathology. 2014, vol. 64, vol. 1, pp. 53-67, ISSN 1365-2559.
- National Cancer Institute (USA). Gastrointestinal Stromal Tumors Treatment: Stage Information for Gastrointestinal Stromal Tumors [online]. [feeling. 4/2014]. < https://www.cancer.gov/types/soft-tissue-sarcoma/hp/gist-treatment-pdq#section/all >.
- DAUM, O. and M. ŠEDIVCOVÁ. Recommended procedure for histological examination of gastrointestinal stromal tumors. Society of Czech Pathologists, 2014.
External links[edit | edit source]
- reGISTer - registry for the collection of epidemiological and clinical data of patients with gastrointestinal stromal tumor
- BEHAZIN, Nancy S .. Medscape: Gastrointestinal Stromal Tumor [online]. © 2013. [feeling. 2014]. < https://emedicine.medscape.com/article/278845-overview ,>.
- CHOTI, Michael. Medscape: Gastric Gastrointestinal Stromal Tumor [online]. © 2013. [feeling. 2014]. < https://emedicine.medscape.com/article/278845-overview >.
- PathologyOutlines.com. Stomach> Stromal / other tumors> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/stomachGIST.html >.
- PathologyOutlines.com. Esophagus> Other malignancies> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/esophagusGIST.html >.
- PathologyOutlines.com. Small bowel (small intestine)> Other malignancies> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/smallbowelGIST.html >.
- PathologyOutlines.com. Colon tumor> Mesenchymal tumors> Gastrointestinal stromal tumors (GIST) of colon [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/colontumorgist.html >.
- PathologyOutlines.com. Appendix> Other tumors> Gastrointestinal stromal tumors (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/appendixGIST.html >.
- PathologyOutlines.com. Liver and intrahepatic bile ducts - tumor> Other malignancies> Gastrointestinal stromal tumor (GIST) [online]. © 2012. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/livertumorGIST.html >.
- PathologyOutlines.com. Stains> DOG-1 [online]. © 2011. [feeling. 2014]. < http://www.pathologyoutlines.com/topic/stainsDOG1.html >.
- ŽABKA, J. Gastrointestinal stromal tumors. Klin Cnkol [online] . 2011, vol. 24, vol. 3, pp. 187-194, also available from < http://www.eonkologie.cz/klinicka-onkologie/archiv/2011/53-archiv/2011-3/248-2011-03zabka >. ISSN 0862-495X.
- DAUM, O, R ŠÍMA and M MICHAL. Pathological diagnosis of gastrointestinal stromal tumor. Oncology [online] . 2010, vol. 4, vol. 1, pp. 13-17, also available from < http://www.onkologiecs.cz/pdfs/xon/2010/01/04.pdf >. ISSN 1803-5345.