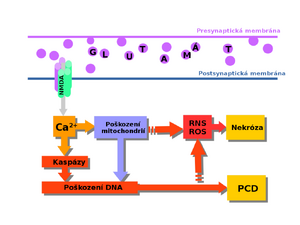
Excitotoxicity in the pathogenesis of CNS disorders
Glutamate and its excitotoxicity[edit | edit source]
Glutamate plays an important role in the development of nervous tissue, its plasticity and during the transmission of excitation signals at synapses. It is essential for the function of nervous tissue, but in large amounts it acts excitotoxically as a nerve poison.
The excitotoxic effects of glutamate are sometimes mentioned in connection with autism. However, its importance in the etiopathogenesis of this disorder is debated.
The effect of hypoxia on the brain[edit | edit source]
See the Hypoxia page for more detailed information .
There are several reasons for the increase in glutamate levels. Hypoxia will serve as a model example, as it is followed by events that result in excitotoxic damage to nerve tissue.
The brain is literally "second to second" dependent on the level of oxygen and glucose in the incoming blood. This dependence is due to the high metabolic activity of nervous tissue, small energy intracellular stores and unconditional dependence on aerobic glucose metabolism. Decreased brain perfusion causes a critical shortage of energy resources. Neurons need more glucose and oxygen than they get. At the same time they are flooded with glutamate. Lack of energy leads to incipient voltage failure, which, if lasted long enough, can result in the failure of vital cell functions and, as a result, cell death.
A decrease in energy sources leads to a decrease in ATP levels, which limits the functions of ion pumps, such as the Na + / K + pump, which is necessary to maintain high intracellular potassium concentrations (155 mmol / l) and low intracellular sodium concentrations (12 mmol / l). . Failure of the pump function leads to a decrease in the electrical gradient on the membrane (depolarization) and the opening of voltage-gated ion channels. A cascade of events is activated, which results in cell death. Depending on the type of involvement, or the proportion of specific cells, there may be either damage to specific groups of neurons that are more vulnerable, or damage to all neurons present in the area, to a stroke.
Immediately after ischemic injury, normal brain activity disappears due to the activation of potassium channels and the subsequent spread of hyperpolarization. This is probably caused by the opening of potassium channels, which is affected by the local concentration of ATP, H + and Ca2 +. Opening may also be associated with the alteration of non-heme metalloproteins and regulation of specific potassium channels[1]. This probably protective reaction is unable to maintain the level of "energy-rich phosphates" and both ATP and creatine phosphate levels fall rapidly in the minutes following the onset of ischemic damage.[2] A decrease in pO2 during ischemia leads to increased lactate production and the cell undergoes a Pasteur shift from dependence on aerobic metabolism to dependence on anaerobic glycolysis.
The resulting lactic acidosis lowers the pH of ischemic tissue from normal 7.3 to values ranging from 6.8 to 6.2. This value depends on the initial state - the amount of glucose that can be converted to lactate.
Potassium leaching secondarily leads to increased extracellular potassium concentrations and massive gradual depolarization, also referred to as spreading depression. Rapid inactivation of O2-sensitive potassium channels may be one of the mechanisms by which nervous tissue prevents the increasing efflux of potassium.[1] Other gradients are also lost.
Change in intracellular ion concentrations:
- the level of Na + and Ca2 + is increased;
- the level of Mg2 + is reduced.
Increased stimulation of the NMDA receptor with glutamate leads to an increase in intracellular calcium levels, which are at the beginning of a cascade of events leading to cell death
The extracellular concentration of many transmitters is increased during ischemia-hypoxia. Depolarization-induced Ca2 + influx through voltage-gated Ca2 + channels stimulates leaching of the cell's vesicular pool, including the excitatory amino acid glutamate. Increased glutamate intake is associated with the intake of 2 Na+ (according to older sources 3 Na+) and the exclusion of K + and HCO3− / OH−.
As the gradient on the membrane gradually decreases, the increased uptake of glutamate is stopped .
Glutamate accumulating in synapses leads to massive stimulation of its receptors, which is usually toxic. Glutamate activates 3 classes of receptors:
- NMDA;
- AMPA;
- Kainate type
These receptors alter calcium ion permeability due to glutamate stimulation (see figure). The ions then trigger a variety of lethal reactions, including nitrosative stress[4].
Disease and therapeutic prospect[edit | edit source]
We consider excessive stimulation of glutamate receptors to be the first cellular response in a stroke. Increased stimulation of NMDA receptors is also found in Alzheimer's disease, where it leads to increased production of APP (amyloid precursor protein) and subsequent accumulation of beta-amyloid. This receptor thus represents an interesting therapeutic target. However, the way in which it would be possible to selectively block cell-associated NMDA receptors and omit other, physiologically indispensable ones remains a problem[5]. The NR2B subunit offers some hope and has recently received increased attention.[5][6] Today, these subunits are thought to be combined with other types, thus limiting possible therapies.[7] One of the possible causes of failure is also a short therapeutic window. Last but not least, we encounter excitotoxic damage in one of the ten known forms of amyotrophic lateral sclerosis, when a mutation in the gene for the production of superoxide dismutase (SOD1, 21q22.11, inherited dominantly and recessively). Loss of SOD1 function then leads to increased oxidative stress, impaired mitochondrial function, RNA destabilization, disruption of synaptic transmission, and glutamate excitotoxicity.
Odkazy[edit | edit source]
Reference[edit | edit source]
- ↑ a b CHIDEKEL, A S – FRIEDMAN, J E – HADDAD, G G. Anoxia-induced neuronal injury: role of Na+ entry and Na+-dependent transport. Exp Neurol [online]. 1997, vol. 146, no. 2, p. 403-13, Available from <https://www.ncbi.nlm.nih.gov/pubmed/9270051>. ISSN 0014-4886.
- ↑ Welch et al., 1997
- ↑ Nicholls et al., 1990
- ↑ AARTS, Michelle – LIU, Yitao – LIU, Lidong. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science [online]. 2002, vol. 298, no. 5594, p. 846-50, Available from <https://www.ncbi.nlm.nih.gov/pubmed/12399596>. ISSN 0036-8075 (print), 1095-9203.
- ↑ a b TU, Weihong – XU, Xin – PENG, Lisheng. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell [online]. 2010, vol. 140, no. 2, p. 222-34, Available from <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2820131/?tool=pubmed>. ISSN 0092-8674 (print), 1097-4172.
- ↑ Hardingham et al., 2010
- ↑ Thomas et al., 2006
Použitá literatura[edit | edit source]
- SIEGEL, George J. Basic Neurochemistry : Principles of Molecular, Cellular and Medical Neurochemistry. 8. edition. Elsevier, 2012. 1093 pp. vol. 1. ISBN 978-0-12-374947-5.
- GOLANOV, E, D REIS, Lisheng PENG, Xiaofen ZHONG, Wenfeng ZHANG, Mangala M. SOUNDARAPANDIAN, Cherine BELAL, Manqi WANG, Nali JIA, Wen ZHANG, Frank LEW, Sic Lung CHAN, Yanfang CHEN a Youming LU. Oxygen and Cerebral Blood Flow: implications for neurodegenerative disorders. Primer on Cerebrovascular Diseases. Elsevier, 1997, vol. 32, issue 12, s. 58. DOI: 10.1016/B978-012743170-3/50016-9. Dostupné z: https://linkinghub.elsevier.com/retrieve/pii/B9780127431703500169
- HARDINGHAM, Giles E., Hilmar BADING, Lisheng PENG, Xiaofen ZHONG, Wenfeng ZHANG, Mangala M. SOUNDARAPANDIAN, Cherine BELAL, Manqi WANG, Nali JIA, Wen ZHANG, Frank LEW, Sic Lung CHAN, Yanfang CHEN a Youming LU. Synaptic versus extrasynaptic NMDA receptor signaling: implications for neurodegenerative disorders. Nature Reviews Neuroscience. 2010-9-15, vol. 11, issue 10, s. 682-696. DOI: 10.1038/nrn2911. Dostupné z: http://www.nature.com/articles/doi:10.1038/nrn2911
- THOMAS, C. G., Hilmar BADING, Lisheng PENG, Xiaofen ZHONG, Wenfeng ZHANG, Mangala M. SOUNDARAPANDIAN, Cherine BELAL, Manqi WANG, Nali JIA, Wen ZHANG, Frank LEW, Sic Lung CHAN, Yanfang CHEN a Youming LU. Synaptic and Extrasynaptic NMDA Receptor NR2 Subunits in Cultured Hippocampal Neurons: implications for neurodegenerative disorders. Journal of Neurophysiology. 2006-03-01, vol. 95, issue 3, s. 1727-1734. DOI: 10.1152/jn.00771.2005. Dostupné z: https://www.physiology.org/doi/full/10.1152/jn.00771.2005?cookieSet=1