Electrolysis
In the interpretation of electrode events , we assumed that the system is in an equilibrium state. Let us now consider what happens if we apply voltage to the electrodes from an external source (electrolytic cell, ΔG > 0). The ongoing events will be governed by Faraday's laws . In order for electrolytic reactions to take place, the voltage on the electrodes must exceed a certain value - the breakdown voltage (corresponds to the sum of the standard electrode potentials for individual reactions).
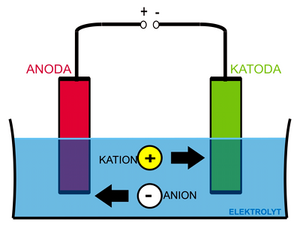
As an example, we will use a system consisting of two inert (platinum) electrodes in an aqueous solution of sodium chloride.
If we apply a voltage to the electrodes, the ions in the solution begin to travel according to their charge, Na+ and H+ cations to the cathode and Cl− and OH− anions to the anode. Reactions will take place at the cathode (and in its immediate vicinity):
- 2 H+ + 2 e− → H2 ↑
and (at higher voltage):
- Na+ + e− → Na
- 2 Na + 2 H2O → 2 Na+ + 2 OH− + H2 ↑
A reaction will take place at anode:
- 2 OH− → H2O + ½ O2 ↑ + 2 e−
and (at higher voltage):
- 2 Cl− → Cl2 + 2 e−
- Chlorine will be partly released as a gas, partly it will further react with water to form hydrochloric acid and hypochlorous acid:
- Cl2 + H2O → H+ + Cl− + HClO
The resulting gases will escape from the system (in this case, it is therefore an irreversible event). We also know the above example from practice - notice, for example, the bubbles at the electrodes during electrophoresis.
Use[edit | edit source]
|
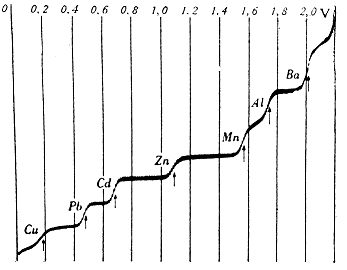
| Polarographic curve, dependence of current on voltage. Taken from [1] |
This phenomenon is also used, for example, in polarography. Simply put, if we gradually increase the voltage across the electrodes, only a small current will initially flow through the system. After reaching the breakdown voltage, characteristic of the respective redox couple, electrolytic processes begin to take place on the surface of the measuring electrode and the current increases sharply (the rising part of the polarographic curve). Since this reaction is very fast, the ions around the electrode will soon be depleted. New ions reach the surface of the electrode by diffusion, the speed of which is limited, so that the intensity of the current does not change much (flat plateau). If we construct a graph of the dependence of the current on the input voltage, we get a step-like polarographic curve.
Links[edit | edit source]
Reference[edit | edit source]
- ↑ CANOV, Michael. Polarografie [online]. [cit. 2018-10-20]. <http://canov.jergym.cz/analchem/polar.htm>.