Disorders of acid-base balance
We refer to conditions when:
- The pH of the internal environment is deviated from the norm (acidemia, alkalemia)
or
- there is an excess or lack of acids or bases in the body, i.e. there is a change in the composition of buffers (which may or may not be accompanied by a change in the resulting pH; acidosis, alkalosis).
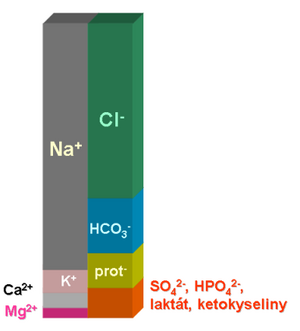
For the rapid maintenance of pH, the 'bicarbonate buffer is of the greatest importance. One of its components, (HCO3-), has a charge and forms a relatively significant item in the ionogram. Acid-base balance is therefore closely linked to major ion metabolism. In practice, every major disturbance in the acid-base balance will also be accompanied by a disturbance in the mineralogram. Conversely, more significant changes in the ionogram are usually accompanied by a disturbance in the acid-base balance. More on the relationship between acid-base balance and ionogram can be found here.
Respiratory disorders of acid-base balance
If ventilation changes, the partial pressure of carbon dioxide in the blood changes and thus the concentration of conjugated acid of the bicarbonate buffer. Specifically:
- hyperventilation accompanied by hypocapnia will lead to respiratory alkalosis
and conversely
- hypercapnia caused by ventilatory failure will result in respiratory acidosis.
Metabolic disorders of acid-base balance
As ABR metabolic disorders, we refer to states in which the concentration of bicarbonates (more precisely: standard bicarbonates - see Acid-base balance examination) changes significantly. At the same time, the concentration of one or more main ions always changes, as the bicarbonate anion must be in balance with the other ions of body fluids (more in the chapter Relationships between acid-base balance and ionogram).
Metabolic acidosis
Metabolic acidosis is a condition in which the concentration of standard bicarbonates falls below reference values. This can happen:
- due to the accumulation of an anion that "pushes" the bicarbonates out of the mineralogram;
- due to the loss of bicarbonates (accompanied by a cation, most likely as sodium bicarbonate);
- more rarely: due to losses of some cations, most likely sodium, which are compensated by a decrease in bicarbonate concentrations.
MAC:
Acidosis due to ESRD or ARF:
MAC 2:
Metabolic acidosis from anion accumulation
- Lactic acidosis
- lactic acid in a medium close to 7.4 dissociates almost completely into the lactate anion. Lactate concentration increases significantly, especially in tissue hypoxia.
- Ketoacidosis
- (in terms of ABR accumulation of β-hydroxybutyrate and acetate). It develops when glucose is not a sufficient source of energy and fats are broken down to an increased extent: during starvation, type 1 diabetes, extreme exercise, etc.
- Renal acidosis
- in renal failure, sulphates, phosphates and other anions accumulate that would normally be excreted in the urine.
- Acidosis in some poisonings
- ethanol intoxication - ethanol is metabolized to acetate.
- In addition to acetate overproduction, NADH production plays an important role in ethanol degradation. The high concentration of reducing equivalents inhibits the breakdown of lactate that accumulates. Similarly, NADH inhibits glycolysis, which ultimately leads to stimulation of ketogenesis and accumulation of β-hydroxybutyrate and acetate.
- methanol intoxication - methanol is metabolized to formate;
- ethylene glycol intoxication - metabolized to oxalate.
Metabolic acidosis from bicarbonate losses
It is most often due to the loss of bicarbonates from the gastrointestinal tract. Duodenal and pancreatic juices are rich in bicarbonates, which are supposed to neutralize the digestion coming from the stomach. Normally, bicarbonates are resorbed back in the small intestine. In some GIT diseases (diarrhea, short bowel syndrome), resorption may be so low that blood bicarbonate levels drop significantly.
Renal loss of bicarbonate may be another cause (renal tubular acidosis, side effect of diuretic therapy, etc.). We can also include the so-called dilution acidosis in the group of metabolic acidoses from bicarbonate losses. It occurs during rapid infusions. Bicarbonates dilute in the blood faster than can be supplemented by metabolism. The processes that maintain the carbon dioxide partial pressure are much faster, so pCO2 does not change.
Renal failure
Metabolic acidosis typically develops in renal failure. There are several disorders that affect the acid-base balance in the same direction:
- accumulation of sulphates,
- accumulation of phosphates,
- hyperuricaemia - uric acid behaves like an anion at a pH close to physiological,
- bicarbonate reabsorption fails while maintaining diuresis and tubular damage.
Metabolic alkalosis
MAL:
Metabolic alkalosis is characterized by an increase in the concentration of standard bicarbonates. In principle, this may be due to:
- losses of an anion, usually chlorides or proteins, which are compensated in the ionogram by the addition of bicarbonates;
- an increase in the concentration of a cation, most often sodium.
Alkalosis from anion losses
- Hypochloraemic alkalosis
- It is accompanied, for example, by prolonged vomiting, in which a large amount of chloride anion is lost through vomiting gastric juice. Diuretics may be another cause of hypochloraemic alkalosis.
- Hypoproteinemia
- Proteins behave like polyanions, so the decrease in their concentration is also compensated by the addition of bicarbonates. Typical examples may be liver proteosynthesis failure, protein loss in nephrotic syndrome, or malnutrition.
Hypernatremic alkalosis
It is most often the result of hyperaldosteronism. Some adrenal tumors or other tumors producing this hormone lead to primary hyperaldosteronism. Secondary hyperaldosteronism is more common as a consequence of liver failure, as aldosterone is broken down in the liver. Another cause of secondary hyperaldosteronism may be overactivation of the renin-angiotensin-aldosterone system.
Elevated aldosterone levels cause the kidneys to retain more sodium, which is compensated in the ionogram by the addition of bicarbonate anion. In addition, sodium is being saved at the expense of increased urinary potassium and proton losses, leading to further deepening of alkalosis.
Sodium retention is also caused by corticosteroids, so metabolic alkalosis is accompanied by Cushing's syndrome.
Alkalosis from an excess of other cations
Rarely, metabolic alkalosis can be caused by an excess of another cation, such as ionized calcium. It occurs, for example, in bone tumors (multiple myeloma, metastases of breast cancer, prostate cancer, etc.). During the breakdown of bone tissue, a large amount of Ca2 + as well as HCO3- is released.
Liver failure
Liver failure is typically accompanied by metabolic alkalosis. Its causes are:
- hypoproteinemia in proteosynthesis failure;
- secondary hyperaldosteronism with sodium retention - aldosterone is normally broken down by the liver;
- slowing down the ureasynthetic cycle - a metabolic process that produces a proton for each molecule of urea formed.
Combined disorders of acid-base balance
In practice, one can often encounter a combination of several disorders of acid-base balance. The combination of metabolic acidosis with metabolic alkalosis' is particularly significant: during ABR examination according to Astrup, the individual parameters may be normal or only slightly deviated. Therefore, the combined ABR disorder may not be recognized or may be underestimated. At the same time, a treatment intervention that affects one of the sub-disorders can cause the other disorder to quickly prevail. This can cause a sharp change in the pH of the internal environment and severe metabolic disruption.
Conditions leading to combined ABR disorders are not rare. Typical examples can be:
- vomiting and diarrhea
- vomiting leads to hypochloremic alkalosis, diarrhea to acidosis from bicarbonate losses
- protracted vomiting
- hypochloremic alkalosis from vomiting is combined with ketoacidosis from starvation and lactic acidosis from insufficient tissue perfusion from hypovolemia
- hepatorenal failure
- hepatic metabolic alkalosis is combined with renal acidosis
- liver failure with respiratory insufficiency
- severe hypoproteinemia in liver failure leads to pulmonary edema, lactic acidosis develops as a result of hypoxia
- renal failure with nephrotic syndrome and severe hypoproteinemia
- renal acidosis from the accumulation of sulfates and phosphates is accompanied by alkalosis in hypoproteinemia
Correction and compensation of acid-base balance disorders
If, for any reason, the ABR is impaired, the organism begins to make efforts to maintain the pH of the internal environment. In essence, ABR fights the original disorder with another disorder that shifts the pH in the opposite direction. We distinguish two groups of such mechanisms:
- Compensation means that in the event of a metabolic disorder, the pH of the internal environment is maintained by changing respiration. For example, metabolic acidosis is compensated by respiratory alkalosis; the patient will take labored deep breaths ("Kussmaul breathing").
- We only talk about ``correction in the case of ABR metabolic disorders: one metabolic deviation is corrected by another. E.g. a patient with liver failure (and thus metabolic alkalosis) will excrete more bicarbonate through the kidneys and will acidify the urine less.
Corrective and compensatory mechanisms take time to develop. A change in respiration occurs almost immediately after the occurrence of an ABR disturbance. Respiratory compensatory mechanisms then deepen, reaching their maximum in about 12-24 hours. Compensation and correction at the level of the kidneys is much slower - because some transport mechanisms have to be reregulated, which often requires protein synthesis. These mechanisms reach their maximum in five days.
When arriving at high altitudes, acclimatization takes about five days. The cause of altitude sickness is hyperventilation, which the body tries to counter hypoxia. Effortful breathing, however, will not greatly improve hemoglobin saturation with oxygen - the partial pressure of O2 in the surrounding atmosphere is too low for this, but it leads to respiratory alkalosis. It is alkalosis and ionic imbalance that is the cause of the manifestations of altitude sickness, including cerebral edema, pulmonary edema and tachycardia. Acclimatization consists in over-regulation of the kidneys - essentially in the development of metabolic acidosis, which lasts the mentioned 5 days. It can be accelerated by taking in a large amount of fluids, as the loss of bicarbonates into the urine will increase. As part of the treatment of altitude sickness, the administration of acetazolamide - a carbonic anhydrase inhibitor, which reduces the acidification of the urine - is sometimes recommended (however, more recent work considers the administration of acetazolamide to be of little effectiveness).
Principles of treatment of acid-base balance disorders
Treatment of metabolic acidosis
The basis of the treatment of severe metabolic acidosis is the administration of sodium bicarbonate, either parenterally as part of complex infusion therapy or orally. The advantage of enteral administration is that the organism is left to regulate the absorption of bicarbonates, so there is no need to worry so much about excessive alkalization. On the other hand, this route is slower and less effective, and resorption may be impaired in patients in a more severe condition.
Milder and chronic metabolic acidosis is often corrected by administration of organic acids and their salts. Bicarbonates are actually created only by metabolizing them in the citrate cycle. A condition is good liver function. Lactate (e.g. lactated Ringer's solution for infusion) and citrate (e.g. in oral rehydration solutions used to treat diarrheal diseases) are most commonly used.
If acidosis and acidemia lasted for a long time, it is necessary to adjust the pH of the internal environment slowly.
It should be remembered that the respiratory center responds to pCO2 as an acid-base sensor: CO2 diffuses from the blood into an environment rich in HCO3< sup>-, so a buffer is created. Its pH depends on the current pCO2. Nerve endings react to the acidity of the environment. With acidosis lasting several days, the respiratory center will be overregulated. A sharp alkalinization of the internal environment could lead to the respiratory center behaving as in hypocapnia - i.e. hyperventilation: breathing would be depressed, even respiratory insufficiency.
Treatment of metabolic alkalosis
The treatment of metabolic alkalosis is most often based on the administration of a physiological solution. While in the blood the concentration of sodium cations is higher than the concentration of chloride anions, in physiological solution both ions are in a 1:1 ratio. By administering a physiological solution, we supply the body with an excess of chlorides. This causes hydrogen carbonates to be displaced in the ionogram and alkalosis is corrected.