Chemically Gated Ion Channels
The change in the permeability of chemically controlled Ion Channel is generally determined by the interaction between the Ligand and its Receptors.
We recognize several sub-subtypes of chemically gated channels:
- channels controlled by hormones and mediators
- ion channels controlled by excitatory amino acids
- ion channels controlled by G-proteins
- chemically gated chloride channels
Hormone-controlled channels and mediators[edit | edit source]
These channels are embedded in the cytoplasmic membrane. The mechanism of their opening and closing is different, but it is always a chemical reaction that can be significantly influenced by the administration of inhibitors. When competitive inhibitors are administered, the formation of a reversible complex with the agonist cannot occur, and therefore the channel will not open. When non-competitive inhibitors are administered, the probability of channel opening is significantly reduced.
Nicotinic (N)-type acetylcholine ion channel[edit | edit source]
It is a glycoprotein consisting of five subunits (α2, β, γ, δ). It is found in the postsynaptic membrane of the neuromuscular junction and in the synaptic ganglia. The released acetylcholine binds to the extracellular parts of both chains, an action potential is generated and muscle contraction is triggered. Nicotine is an agonist. The receptor reacts to it quickly, but for a short time. Mainly Na+ and K+, ions can pass through the open channel , but also divalent cations (mainly Ca2+) and to a limited extent also histidine and choline. Chloride ions do not pass through this channel. Blockage of the channel is possible by a number of organic substances. E.g. arrow poison (curare), snake venoms (bungarotoxin) and various other substances including some narcotics. Furthermore, it is possible to block the channel of Mg2+ ions.
Muscarinic (M)-type acetylcholine ion channel[edit | edit source]
It has a similar protein structure to N type, but different functional properties. The receptor exhibits a long latency (100ms) followed by a long effect (300-500ms). We recognize several M subtypes:
- M1
- found in parasympathetic nerve endings. After activation, the production of IP 3 (inositol triphosphate) and DAG (diacylglycerol) increases, and subsequently the concentration of intracellular Ca2+.
- M2
- found in the myocardium aand smooth muscle. Upon activation, the formation of cAMP (cyclic adenosine monophosate) decreases and K+ channels opening.
- M3
- it is found in exocrine glands, smooth muscle and in the endothelium of blood vessels. After activation, the concentration of Ca2+, increases in the cell , which activates contraction in the smooth muscle. Various exocrine glands are also stimulated, secretion production in the sweat, salivary glands and lacrimal glands increases.
- M4 and M5
- they are located in the CNS and their role has not yet been precisely elucidated.
Ion channels controlled by excitatory amino acids[edit | edit source]
These channels are found at the excitatory synapses of most CNS neurons. They are influenced by L-glutamic and L-aspartic acid. We can distinguish two types of glutamate receptors:
- activated by N-methyl-D-aspartic acid (NMDA receptors)
- activated by kainic acid (non-NMDA receptors).
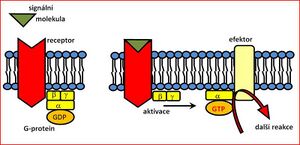
G-protein-gated ion channels[edit | edit source]
This is probably the most complex group of channels on the membrane, which are not only affected by the action of neurotransmitter or hormone but also by various smells, the effect of light and a whole range of other factors. Membrane proteins bind GTP (guanosine triphosphate) - they are therefore called G-proteins.
There are two categories of G-protein:
- stimulatory G-proteins (inhibited by pertussis toxin)
- inhibitory G-proteins (inhibited by cholera toxin)
These G-proteins regulate not only ion channels, but also a number of metabolic events in the cell. They affect, for example: gene expression, cell growth, and [[cytoskeleton] composition. The mechanism of their effect is complex:
- indirect effect – activation of various signaling systems (via second messengers)
- direct effect – acting on various Ca2+, Na+ and K+ channels.
Chemically gated chloride channels[edit | edit source]
Chemically controlled chloride channels are part of both the postsynaptic membrane of inhibitory neurons in the CNS (their activation and opening is the basis of postsynaptic depression) and axo-axonal synapses of primary afferent fibers (activation is the basis of presynaptic depression). The opening of chloride channels causes a depolarization of the cell in most Neurons. Chloride channel inhibition is caused by two amino acids. γ-aminobutyric acid (GABA) and glycine. GABA, which is a product of glutamate decarboxylation, acts mainly in the higher CNS sections or the endings of primary afferent fibers. Glycine acts primarily on motor neurons in the spinal cord.
Related Articles[edit | edit source]
Links[edit | edit source]
Source[edit | edit source]
- MATOUŠ, Bohuslav, et al. Fundamentals of medical chemistry and biochemistry. 2010 edition. Prague: Galen, 2010. 540 pp. ISBN 978-80-7262-702-8 .
Recommended Reading .[edit | edit source]
- MATOUŠ, Bohuslav, et al. Fundamentals of medical chemistry and biochemistry. 2010 edition. Prague: Galen, 2010. 540 pp. ISBN 978-80-7262-702-8
- MURRAY, Robert K. (Robert Kincaid). Harper's biochemistry. 25th edition. New York : McGraw-Hill, c2000. 540 pp. ISBN 08-385-3684-0