Spectrophotometry (2. LF UK)
(Redirected from Spectrophotometry Practical)
|
Check of this article is requested. Suggested reviewer: Carmeljcaruana |
Introduction[edit | edit source]
Spectroscopy is the study of the entire electromagnetic spectrum as well as its interactions with matter. It examines relations of absorption, emission or dispersion of electromagnetic radiation by matter as a function of wave length (λ) or frequency (f). It can be used to identify substances and measure their concentrations. The latter is called spectrophotometry. Devices which measure in one or few specifically defined wavelengths of monochromatic light are called photometers.
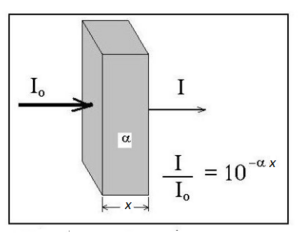
When a light beam of intensity (Io) is passed through a solution a fraction of the photons are absorbed or scattered and the exiting beam has a lower intensity (I). The Lambert’s law (see fig 1) applies where x is the thickness of the attenuating solution and alpha is called the linear attenuation (or absorption) coefficient.
The ratio I/Io is known as transmittance (T). Its values ranges from 0 (all light was attenuated) to 1 (all light passed through; if fluorescent substances are being used, it can reach values higher than 1).
The absorbance (A), defined in fig2, is a function of wavelength (or frequency). The variation of absorbance with wavelength is called the absorption spectrum.
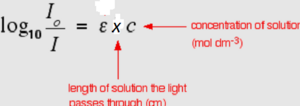
For absorption in solution, Beer's law says that the linear attenuation (or absorption) coefficient is proportional to the concentration. Since the concentration (c) is molar in most cases ε the constant of proportionality is called the molar attenuation (or absorption or 'extinction') coefficient. It is specific for every individual substance and wavelength.
When combined, these two relations form the Beer-Lambert law A = ε · x · c (fig 3) where x is the length of the cuvette.
Spectroscopic properties in visible (VIS) and ultraviolet (UV) are studied in spectroscopy and photometry. They use light the UV area (wavelength 10-390 nm), visible areas (wavelength 390-790 nm) and adjacent infrared areas (wavelength 770 nm - 1 mm). Thanks to the Lambert-beer’s law, it is possible to determine concentration of unknown solutions.
Measuring ε and unknown concentrations for a given substance using a calibration curve method: The absorbance of several calibration samples of known concentration of the given substance is measured. All samples have different concentrations but are placed in the same cuvette and the same wavelength is used to measure them. By drawing a graph (calibration curve) of log10 Io/I against the known concentrations c, ε can be obtained from the gradient (linear regression). this method is suitable for simple solutions. We must pay attention to the units because concentration is in micromole per liter (μMol/L), while the width of cuvette is in centimetres. Once ε is known we can find the concentrations of unknown solutions.
In practice the validity of Lambert-Beer law is limited by the dispersion of light because of tiny impurities in the sample; phosphorescence or fluorescence of the sample; small amount of light passing through highly concentrated solutions; changes of values of absorbing coefficients; shifting chemical equilibrium caused by high concentration of substances in the sample.
Measuring Principle[edit | edit source]
Spectrophotometer is made of these parts
• Light source (halogen light for visible light, deuterium for UV area)
• Lenses and mirrors that direct the beam of light
• Monochromator (device transmitting light of a given wavelength only) – Nowadays optical grids are usually used. which allow the wavelength to change fluently.
• Cuvette space – Space for samples in cuvettes that can be made from plastic, glass or quartz glass
• Light sensor (charge-coupled device or photodiode) – The precision of the measurement depends on the integration time – the time over which the light is measured, the longer the more precise but the longer the measurement which is very important when processing a larger number of samples, when using larger number of wavelengths or when processing samples which are changing over time (kinetic measurements).
• Output device (software for data analysis)
Based on the number of beams used for measuring, we distinguish single or double-beam spectrophotometers. Double-beam spectrophotometers use one beam for measuring the sample, and the other for a blank (i.e., cuvette filled with solvent without dissolved substance so that on can eliminate the attenuation of the solvent itself by subtraction). When using the single-beam device we must first measure blank and then measure the sample solution.
Modern spectrophotometers are fully automatized and controllable via computers. They are used for measuring at one or more measurements or even whole absorption spectra (wide spectrum of wavelengths). They can also be used for measuring kinetics of simple, for example enzymatic reactions. Thus, it’s used in a wide range of scientific disciplines such as chemistry, physics, biochemistry, biology, biophysics and even medicine.
Importance in clinical medicine[edit | edit source]
Spectrophotometers are of high clinical importance in almost all branches of medicine. Their ability to measure concentrations of metabolically important substances in body fluids, such as blood, cerebrospinal fluid, urine and amniotic fluid among others, is crucial for correct diagnostic findings and continuous monitoring of patients. The substances that can be quantitatively analyzed by spectrophotometers are numerous. They include: hemoglobin, erythrocytes, hematocrit, amylase, bilirubin, cholesterol, glucose, urea, creatinine, lipase, triglyceride, albumin, alcohol, ammonia, copper, magnesium, lactate, calcium, iron, magnesium, aluminium, sodium carbonate, carbon monoxide and even certain enzymes. Early identified small deficiencies of certain essential substances might help prevent associated health problems.
In intensive medicine the use of spectrophotometric analyses is more frequent, due to the fact that patients in unstable states are more prone to drastic changes in the amount of different substances in, for example, their blood. Intensive care units therefore frequently have spectrophotometers on site whereas general practitioners might send their probes to be analyzed in a laboratory.
Literature review[edit | edit source]
What are its advantages and disadvantages?[edit | edit source]
Can measure the concentrations of a lot of substances.
An inconvenience of using spectrophotometry is that measurements have to be taken meticulously. Any trace of fingertips or condensation on the cuvettes can create random or systematic error in measurements, and therefore the use of microfiber paper is recommended (Musiol). Microfiber can remove any dust on the cuvettes. It also does not create small abrasions on the cuvette. Another limitation to the precision of spectrophotometers can be the precision of the tools used to make the calibration concentrations such as pipettes.
How does it work?[edit | edit source]
A spectrophotometer consists of 6 basic components. A light source is needed, generally a deuterium lamp and a Halogen lamp are used. The use of two different sources of light provides the device with wider wavelengths of light and therefore a more versatile use. Then the light can either go through a monochromator containing diffraction grating or not, depending on the nature of the measurement. A monochromator will enable only a certain wavelength (in nm) to go through the aperture.
In case no monochromator is used, a light containing all visible wavelengths is emitted and goes through the sample. The light then exits the sample, enters a closed compartment through the exit slit and hits the light sensor (e.g., charged coupled device CCD). The CCD will have a certain number of light sensors that will measure the light. The information provided by the CCD will then be transferred to a computer software which will be able to read and display the transmittance of a compound at MULTIPLE wavelengths. The CCD needs to be calibrated posteriorly to the measurements with a reference cuvette of distilled water for example. This reference cuvette will indicate to the software the amount of LIGHT ABSORBED BY THE SOLVENT AND CUVETTE MATERIALS ONLY and at all the wavelengths of visible light.
Are there any risks involved in it’s use (for patients and the clinical staff)?[edit | edit source]
The use of spectrophotometers is safe. The deuterium lamp represents no danger as it is filled with Hydrogen gas. Even if certain Spectrophotometers use UV light, it is enclosed and therefore cannot harm us.
Are there ethical issues associated with the topic?[edit | edit source]
There are no real ethical issues associated with spectrophotometry.
The equipment[edit | edit source]
- Spectrophotometer
- Cuvette
- Blank solution
- Computer
- Unknown solution
The methodology[edit | edit source]
The SPECORD 40 spectrophotometer is a single-ray spectrophotometer operating in the wavelength range from 190 to 1100 nm (using a deuterium lamp for the 190–300 nm range and a halogen lamp for the 300–1100 nm range). It is used for measuring absorbance values in the interval of A values from –3 to +3. It can be controlled from the main panel or using the WinAspect program which simultaneously permits simple and rapid data analysis.
Chemicals[edit | edit source]
The following chemicals are used for measurements in this spectrophotometric task:
- Copper sulfate pentahydrate has an absorption maximum in the infrared range.
- Cobalt chloride hexahydrate has an absorption maximum in the green region.
- Nickel sulfate hexahydrate has absorption maxima in the purple and red regions.
- Distilled water, which is used as a reference sample (blank) and for rinsing the cuvettes.
Work with solutions in gloves and goggles, do not inhale or ingest.
Samples[edit | edit source]
Samples labeled A, B and C for measuring spectra are prepared in bottle. Series of solutions of known concentrations marked A1, A2, A3, A4 and A5 for the calibration curve are prepared and a sample of unknown concentration marked AX for determine the concentration by spectrophotometry . Prepare the cuvettes with the reference sample (distilled water) and the individual samples and place them in the prepared cuvette stand before starting the measurement. The individual cuvettes should be filled with samples so that they are approximately to the top of the narrowed part and there is no need to fill them to the top.
Cuvettes insertion[edit | edit source]
Before inserting the cuvette, gently wipe off any excess liquid and remove any dirt on its surface. Open the sample compartment. Insert the cuvette into the cuvette holder. Insert the cuvette as close as possible to the wall of the holder so that the light beam passes through the wider part of the narrowed bottom of the cuvette. Then close the sample compartment door. Make sure the cuvette is vertical in the holder! The spectrophotometer is a device sensitive to liquid spills. Therefore, work carefully. In case of liquid spillage, carefully dry the spilled area!
Tasks[edit | edit source]
1st Task[edit | edit source]
In this task, measure the spectrum of samples A, B and C in the wavelength range 380-1100 nm. Identify individual absorption maxima. Then, based on the number of absorption maxima, determine which chemical (see Chemicals) is represented by each solution A, B, and C. Report the wavelengths and corresponding colors in the visible spectrum for each maxima. For the next task, determine the best wavelength (when the maximum absorption is) for Solution A to use when measuring the calibration curve and determining the concentration of the unknown sample in the next task. Upload the generated pdf file in WinASPECT to the moodle server with the protocol.
Detailed instructions for program control when measuring spectra A, B and C are in the following file.Instructions for measuring spectra
2nd Task[edit | edit source]
Using solutions of known concentrations A1, A2, A3, A4 and A5, create a calibration curve and record the data in the protocol (individual concentrations and absorbances, coefficient of the linear equation, coefficient of determination). From the linear regression values for the calibration curves, calculate the molar absorption coefficient ε and report its value and the formula you used. Then measure the concentration of the unknown sample AX. Use the calculated molar absorption coefficient ε and the measured concentration of the sample AX and calculate the corresponding absorbance, giving its value in the protocol and the formula you used. Finally, compare the measured absorbance and the calculated absorbance. Upload the generated pdf file in WinASPECT to the moodle server with the protocol.
Detailed instructions for operating the program when measuring concentration are in the following file.Instructions for measuring concentration
Conclusion[edit | edit source]
The future of spectrophotometry lies especially in the improvement of pathological diagnostics, disease detection and general clinical research as “uv-vis spectroscopy enables safer, non-invasive analysis of soft tissue, and can enhance accuracy and speed in clinical diagnostics and medical research.”
Around 10% of cancers are due to the exposure of radiation, therefore, such improvements will aid us in the advancement of clinical applications relating to various types of cancers. Spectrophotometry has enabled us to “convert measured reflectance and fluorescence spectra from tissue to cancer-relevant parameters such as vascular volume, oxygenation, extracellular matrix extent, metabolic redox states, and cellular proliferation.” Pulse oximetry is a method of examining a person’s oxygen saturation and it uses in vivo spectrophotometry. This relatively recent method of examination also presents several possibilities for future developments of spectrophotometry by providing “reliable, objective, and non-invasive” results.
Bibliography[edit | edit source]
How Does a Spectrometer Work?" B&W Tek. B&W Tek, 2016. Web. 12 Dec. 2016.
[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2742920/>. Brown, J. Quincy, Karthik Vishwanath, Gregory M. Palmer, and Nirmala Ramanujam. "Advances in Quantitative UV-Visible Spectroscopy for Clinical and Pre-clinical Application in Cancer." Current Opinion in Biotechnology. U.S. National Library of Medicine, Feb. 2009. Web. 19 Dec. 2016.]
“Manuelle Photometer.” n.d. Web. 25 Dec. 2016.
Applications of absorption spectroscopy (UV, visible). 2008. Web. 25 Dec. 2016.
2015. Fabulously versatile uses of a spectrophotometer. Buzzle, 29 Sept. 2016. Web. 25 Dec. 2016.
Translation of wikiskripta:Spektrofotometrie pro praktická cvičení 2.LF UK (3 Dec. 2018)