Spectrophotometry (2. LF UK) OLD
The theoretical part:[edit | edit source]
- Spectroscopy is a physical-chemical discipline that deals with the origin and properties of all kinds of spectra, interactions between matter and electromagnetic radiation. It examines the absorption, emission, or scattering dependences of electromagnetic radiation as a function of wavelength or frequency. The result of the investigation is a curve called substance spectrum, by interpreting which we can obtain information about the quantity and quality of the investigated substances.
- UV/VIS spectroscopy and photometry are disciplines using light from the UV region (wavelength 10-390 nm), the visible region (wavelength 390-790 nm) and adjacent infrared regions (wavelength 770 nm–1 mm) to investigate spectroscopic properties.
- Spectrophotometry it is based on the interaction of electromagnetic radiation with the analyzed solution, when part of the radiation is absorbed by the sample particles. Absorption of a photon changes the energy of the molecule and creates an excited atom, where part of the radiation passes through the solution and is subsequently detected by the device. The amount of light emitted, reflected or absorbed by a certain substance is dependent on the wavelength of the radiation and the concentration of the substance under investigation. Since the intensity of the transmitted light (I0) is always smaller than the intensity of the light incident on the substance (I), the quantity transmittance (T ) describing just this attenuation. It can take values from 0 (all radiation absorbed) to 1 (all radiation passed; for fluorescent substances it can be greater than 1) and is sometimes given as a percentage:
- T = I/I0
- In spectrophotometry, we also encounter a quantity characterized by the negative logarithm of transmittance called absorbance (A). The logarithmic function is used in the formula due to the exponential decay of light intensity as it passes through the substance:
- A = - log10(I/I0)
- The result of the spectrophotometric measurement is the so-called absorption spectrum, which characterizes the properties of the environment. It is a graph of absorbance or energy versus wavelength. The displayed curves are characterized by their shape and the position of the maximum. (Measurement of absorption spectra - see Practical part -Task 1.)
- Spectrophotometry is based on Lambert-Beer's law, which describes the relationship between the concentration of a substance in a solution and its absorbance:
- Aλ = - log10(I/I0) = ελ.l.c = k.c
- It follows that, at a given wavelength, the absorbance is directly proportional to the concentration of the substance c [mol.l-1] and the thickness of the absorbing layer l [cm] , i.e. the width of the cuvette (usually 1 cm). Molar extinction (absorption) coefficient ε [l.mol-1.cm-1] is a proportionality constant that is specific to a given substance and wavelength. We can find out its values from physical-chemical tables or by measuring the absorbance of solutions of known concentrations and creating a calibration curve (see Practical part - Task 2), or by determining the calibration coefficient .
- It also follows from this relationship that for the measurement of the same analyte at the same wavelength and in the same cuvette, we can replace the product (ελ.l) by the constant k. From the measured absorbance of the sample and the calculated constant k, the substance concentration of the unknown sample can then be determined: cvz = Avz/k.
- The Lambert-Beer law applies when monochromatic light is applied, the cuvette length is constant, the solvent is the same, and the substance concentration is constant throughout the measurement. In practice, the validity of this law is limited by light scattering due to fine impurities in the sample, phosphorescence or fluorescence of the sample, small amounts of transmitted light at high concentrations, changes in absorption coefficient values, and shifts in chemical equilibrium caused by a high concentration of a substance in the sample. Other conditions for using the Lambert-Beer law are a low concentration of the substance (on the order of less than 10-2 mol/l) and also that the studied substance does not participate in chemical equilibrium.
- In practice, the Lambert-Beer law allows us to determine the unknown concentration of substance solutions in spectrophotometric measurements. This is determined in two ways: the calibration curve method (see Task 2) and the standard addition method.
- The calibration curve method is a procedure where the absorbance of several calibration solutions of different concentrations is measured in the same cuvette and at the same wavelength. From the course of the calibration curve, we can verify the validity of the Lambert-Beer law. The resulting dependence should ideally be linear, passing through the origin - the so-called calibration line. However, this method is only applicable for a simple matrix (e.g. drinking water).
- The standard addition method is a procedure during which the absorbance of an unknown sample is measured Avz and then the concentration of the substance in the sample is increased by the defined addition of the standard and the corresponding absorbance of the solution is measured. With this method, it is possible to determine a substance in a complex matrix (e.g. in waste water), when determination by the calibration curve method would be quite complex.
- Using the isosbestic point: If a substance that has a certain spectrum changes during a reaction to another substance that has a different spectrum, but the two spectra partially overlap. An example can be the spectra of NAD+ and NADH (the determination of these coenzymes is used when measuring the activity of a number of enzymes using the so-called Warburg optical test). In this case, the absorption maxima are far enough apart that the determination of the individual forms of the coenzyme is simple. However, the fact that all the spectra for different NAD+/NADPH ratios (at constant total concentration) cross over may be useful. This is because at 281 nm both NAD+ and NADH have the same extinction coefficient. The intersection of the spectra is called the isosbestic point (from the Greek ισος isos = same and σβεννυμι sbennými = I extinguish) and by measuring the absorbance at this point we can easily determine the total concentration of NAD+ and NADH without needing to know the actual concentration ratio of the two components.[1]
- Use in medicine: "Spectrophotometry of cerebrospinal fluid" is used in the diagnosis of sudden cerebrovascular events, especially when bleeding into the subarachnoid space is suspected. It provides information on the age of bleeding and on prolonged or repeated bleeding. Spectrophotometric examination of cerebrospinal fluid in the visible part of the spectrum allows oxyhemoglobin, methemoglobin and bilirubin to be characterized on the basis of different absorption maxima. Since spectrophotometric measurement does not damage the measured substances, this technique is also used very often in various biochemical experiments (for example with DNA/RNA and various proteins), when these substances are usually not obtained in large quantities.
- Spectrophotometers are technically more complex and more perfect instruments fully controllable by means of a computer for spectrophotometric determinations, which allow the wavelength of monochromatic light to be set arbitrarily or to measure a part of the absorption spectrum in a certain section of wavelengths. They are used to measure absorption spectra or to determine quantitative measurements, but they also enable the measurement of the kinetics of simple, for example, enzyme reactions. They are widely used in the fields of chemistry, physics, biochemistry, biology and medicine.
- Spectrophotometers are divided according to the number of beams into:
- Single-beam spectrophotometers only measure the outgoing radiation flux, so a reference measurement of the solvent must first be made before the sample can be measured.
- Two-beam spectrophotometers measure a blank sample, the so-called BLANK (solvent without substance to be determined), with one beam, while the sample under investigation is measured with the other beam. Single-beam spectrophotometers are simpler in design and smaller in size, and in some cases connect directly to microscopes.
- Parts of a spectrophotometer:
- Radiation source - incandescent or halogen lamp for VIS and deuterium lamp for UV region
- Lenses and Mirrors - direct the beam of light
- Monochromator - a device that transmits only light of a certain wavelength (makes polychromatic radiation monochromatic); the decomposition element can be a prism or an optical grating (a glass prism is most often used for the visible region of the spectrum)
- Cuvette space - space for samples in cuvettes; glass optics (for VIS range), quartz optics (for UV range), salt optics (for IR range) or plastic cuvettes are used
- Detector - CCD camera or photodiode
- Output device - data analysis software
- The spectrophotometer SPECORD 40 is used in practice. It is a single-beam spectrophotometer that measures in the wavelength range 190-1100 nm (for 190-300 nm a deuterium lamp and for 300-1100 nm a halogen lamp). It enables the measurement of absorbance in the interval of values A = (−3)–(+3). It can be controlled from the main panel on the instrument as well as with the WinASPECT program, which simultaneously allows simple and fast data analysis.
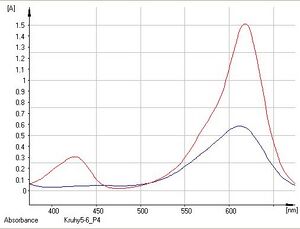
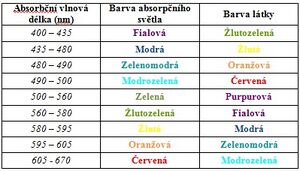
- Solutions appear colored to us because they absorb a certain wavelength of the visible spectrum. The color of the solution is then given by the complementary color to the absorbed color. The absorption spectrum of the substance shows the ability of the particles of a substance to absorb certain wavelengths of electromagnetic radiation. In practice, you will try measuring the absorption spectra of malachite green and indigotine (indigo carmine). Malachite green is a dark green organic dye that is used, among other things, for bacteriological staining or as a local antiseptic. It absorbs in the blue and red regions of the spectrum, which is why it appears green to us. Indigotin (indigocarmine) is a substitute for the natural dye indigo. It is an organic dye that absorbs mainly in the red region of the spectrum, which is why it appears blue to us.
- <mediaplayer width="500" height="300">https://www.youtube.com/embed/pxC6F7bK8CU</mediaplayer>
Practical part:[edit | edit source]
SPECORD 40 spectrophotometer. A single-parse spectrophotometer that measures in the wavelength range 190-1100 nm (for 190-300 nm a deuterium lamp and for 300-1100 nm a halogen lamp). Allows measurement of absorbances in the range of values of A = (–3)–(+3). It can be controlled from the main panel on the instrument as well as with the WinASPECT program, which simultaneously allows simple and fast data analysis.
Malachite green it absorbs in the blue and red regions of the spectrum (the region of visible radiation).
Indigotine (Indigocarmine) it only absorbs in the red region of the spectrum (the region of visible radiation).
Task 1: Measurement of the absorption spectrum of malachite green and indigotine solutions[edit | edit source]
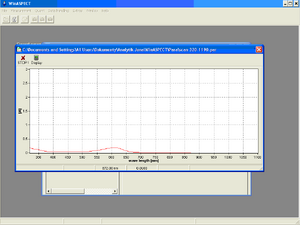
- The task of the first task is to investigate how substances absorb visible light (their absorbance) depending on its color (wavelength) and to draw up a graph of this dependence (absorption spectrum). We then analyze this graph - we determine the wavelength at which the measured absorbance is the highest, therefore the substance absorbs electromagnetic radiation the best. The measurement is carried out for wavelengths in the range of 375–675 nm.
- Saving files and their names
- Save all files created during the practical part of this task in the directory Praktikum_CZ (you can find the link on the desktop). As the name of the file, always say your group name - the first character is the number of your circle and the second is the letter of your team (eg circle 9 group C => the name will be 9C).
- Safety notice
- • Work with solutions in gloves, do not inhale or ingest.
- • A spectrophotometer is a moisture-sensitive device. Be careful with liquids! In the event of a spill, dry the spilled area immediately!
- Chemicals:
- KA4 and KB solutions
- Distilled water
- Workflow
- 1. Turn on the spectrophotometer using the power switch on the back of the instrument
- 2. Turn on the PC
- 3. Start the WinASPECT program (START/PROGRAMS/WinASPECT/WinASPECT)
- 4. After starting the program, open the "Measurement" panel on the top panel and choose "Initialize device". After confirming the choice, a question about the use of a UV lamp will appear, which we will reject. The spectrophotometer automatically starts initialization and internal calibration. Panel Settings
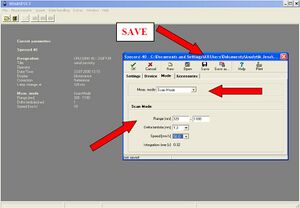
- 5. After the initialization is complete, set the measurement parameters. Select Set Parameters from the Measurements menu. The parameter settings panel will appear. You click on New in the parameter settings panel.
- The parameter settings
- We will set the measurement parameters in the WinASPECT program. The measurement interval is 1 nm, which means that specific absorbance values are recorded for each wavelength in our range with a given difference (for 375, 376, …, 675 nm). The measurement speed is 50 nm/s, so the measurement of one sample takes only 6 seconds.
- Some parameters are preset. To set the measurement correctly, set the following:
- Settings panel:
- • In the "Title" window, fill in the title according to the name of your group (instructions given above).
- ◦ Cycle Mode disabled by selecting None
- ◦ Display function - select the Absorbance option
- ◦ Correction function - select the Reference option
- Mode Panel:
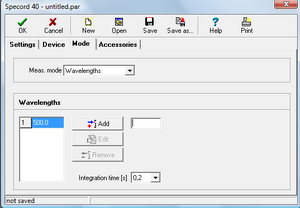
- • In the Meas. Mode select Scan Mode
- • To set the Scan Mode function, set the following values:
- ◦ Range = 375– 675 nm (selection of the monitored spectrum width)
- ◦ Delta lambda = 1 nm (selection of measurement interval length)
- ◦ Speed = 50 nm/s (selection of measurement speed) (so the measurement of one sample takes only 6s).
After setting the parameters, click the OK icon on the measurement settings panel. A save option window will appear. Save the file in the above directory under your group name. After saving, close the measurement settings window.
6. The spectrophotometer is now ready for its own measurement. The Measurement panel is now open. Select the Serial measurement option.
7. The Serial Measurements window is now active in WinASPECT. To set the measurement parameters, select the Setup option from the Edit menu in the toolbar.
Set the following parameters:
• Panel General: Descriprtion: enter the name of your group.
• Panel Samples: Number of measurements: enter the number of samples (2 in this case).
| Sample number | Sample name | Concentration |
|---|---|---|
| 1 | KA4 | |
| 2 | KB |
8. Before measuring the samples, it is necessary to perform a reference measurement using a blank sample, the so-called blank (solvent without the substance to be determined). The samples - dyes - are dissolved in distilled water, so use distilled water as a blank. Pour distilled water into the cuvette so that its level reaches 1/2 of the cuvette (approximately to the top of the narrow part). When measuring samples with different concentrations, it is best to proceed from the most diluted solution to the most concentrated, then we do not need to wash and dry the cuvette (e.g. with pulp) after each measurement.
Always hold the cuvette by the frosted sides. The light beam passes through the clear sides, and their contamination (including fingerprints) can distort the measurement results. Therefore, before measuring, wipe the cuvette dry and at the same time wipe off any dirt and fingerprints (e.g. with dry pulp).
9. Open the sample loading area. Insert a cuvette with a blank (distilled water) into the cuvette holder. Then close the sample compartment door. We insert the cuvette as close as possible to the wall of the holder, so that the light beam can pass through the clear surfaces. Make sure the cuvette is vertical in the holder!
A spectrophotometer is an instrument sensitive to liquid spills. Therefore, work carefully. In case of liquid spillage, carefully dry the spilled area!
10. Start the reference measurement by clicking on the Reference icon in the toolbar of the Serial Measurement window. The spectrophotometer measures the absorption spectrum of the blank.
11. After performing the reference measurement, the spectrophotometer is ready for sample measurement. Start the sample measurement by clicking the Start icon. Pour the first sample (KA4) into a clean cuvette and place the cuvette in the cuvette holder (see points 8 and 9). We will use the most concentrated solutions of dyes, because according to the Lambert-Beer law, the concentration is directly proportional to the absorbance, and therefore the resulting spectrum curve will be more pronounced. Close the lid of the spectrophotometer and confirm the sample insertion in the program ("OK"). The spectrophotometer measures the absorption spectrum of the first sample.
12. After finishing the measurement of the first sample, insert the second sample into the cuvette and confirm the insertion again.
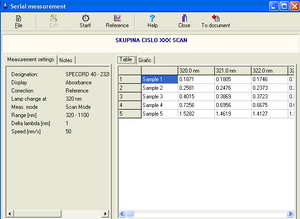
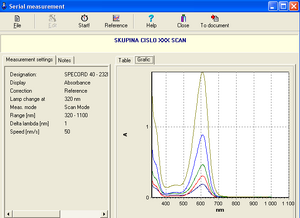
13. After all samples have been measured, a table containing the measured absorbance values at a given wavelength (Table panel) or a graphical representation of the values in the table (Graphic panel) is displayed.
14. Click the To document option on the toolbar of the Serial measurements window to convert the results into a document. Save it under your group name. The document allows viewing of tabulated values as well as absorbance and transmittance values relative to wavelength.
15. We will determine the "ideal" wavelengths for indigo and malachite green solutions (the wavelengths at which their measured absorbances are highest) and print the absorption spectra.
Problem 2: Determination of the concentration of an unknown sample and the Lambert-Beer law[edit | edit source]
The goal of the second task is to determine the unknown concentration of a solution of one of the dyes. According to the Lambert-Beer law, the dependence of absorbance on concentration is linear in a certain range of concentrations. Before measuring unknown samples, it is necessary to determine the coefficient of proportionality. Therefore, we construct a calibration curve by measuring the absorbance of samples of known concentration.
Workflow:[edit | edit source]
Chemicals:
Four indigotine solutions of different concentrations (solutions KA1, KA2, KA3, KA4), a solution of unknown concentration (NA).
Workflow:
Calibration curve
| Sample number | Sample name | Concentration [μM/l] |
|---|---|---|
| 1 | KA1 | |
| 2 | KA2 | |
| 3 | KA3 | |
| 4 | KA4 |
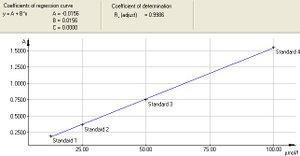
First, we construct a calibration curve, which shows the dependence of the absorbance measured by the instrument on the concentration of the solution. To determine it, we need four indigotine solutions of different concentrations (12,5; 25; 50; 100 µM/l). Based on the calibration curve, the program calculates the values needed to calculate the molar extinction coefficient and finally determines the unknown concentration of the sample in the cuvette.
1. In WinASPECT, choose Calibration from the Quant menu on the toolbar.
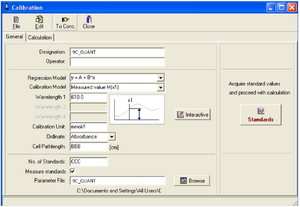
2. A Calibration window will appear on the screen to set the measurement parameters.
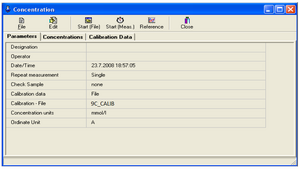
The General area:
• Group name (Designations): the name of your group
• Regression Model (Regression Model): choose an option: y = A + B*x
• Wavelength (WAVELENGHT1) (best fit common to KA4 and KB solutions): choose based on the measured values in task 1.
• Concentration units (Calibration Unit): choose according to the units indicated on the samples
• Measured quantity (Ordinate): absorbance
• Cuvette width (Cell Pathlenght): measure the width of the cuvette
• Number of standards (No. of Standards): 4 for KA
• Parameter file: 9C.par (example - 9 - Ninth Circle, C - Team C)
3. In the Mode panel from the Meas menu. mode select the Wavelenghts option. Select the appropriate wavelength by clicking on the Edit option. Next, write the wavelength value and click the Edit icon again to confirm. Click the OK icon to return to the Calibration menu.
4. After returning to the "Calibration" menu and entering the above parameters, we click on the "Standards" icon.
5. Clicking on the Standards icon will open a window; fill in the concentrations of each standard in the appropriate units.
Standard: fill in the names of the standards
Conc.: fill in the concentration values in the appropriate fields
Source: value entered automatically
6. Measure the absorbance of the reference sample (blank). Place the cuvette filled with distilled water into the cuvette holder and click the Reference icon to perform a reference measurement.
7. Click the Start icon and insert the KA1 sample. Then measure samples KA2, KA3, KA4 in the order given in the table. Click the OK icon when all samples are finished measuring.
8. The Calibration window opens with a graphical representation of the calibration curve and the equation of the regression line.
9. Save the file via File and Save as under your group name. Print the calibration curve via File and Print. When you click on Print, you will see the window of Print - Calibration. In the window, keep only the first four options (Calibration curve, Parameters, Standards and Statistics) checked. If the last option Audit trail is checked, uncheck it. Start your own printing by clicking the Print icon in the Print - Calibration window.
The calibration curve can also be saved after clicking the icon To Conc.
Determination of unknown sample concentration (for NA sample)
10. After saving, the program will automatically open the Concentration window, which allows you to determine the concentrations of unknown samples based on the calibration curve.
11. To measure concentrations of an unknown sample, insert the sample in the cuvette into the cuvette holder and click the Start (Meas.) icon, the instrument will measure the absorbance and calculate the concentration of the unknown sample based on the coefficients of the calibration curve.
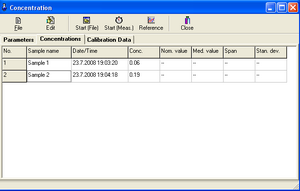
12. After the measurement is finished, the concentration values are displayed in the table.
Table - measuring the concentration of an unknown sample
13. Save the document under the name of your group and record the measured concentration value in the log.
Save the document
14. After finishing the measurement, rinse the used cuvettes with distilled water from the syringe.
Video tutorial[edit | edit source]
<mediaplayer width="500" height="300">https://www.youtube.com/embed/KXJ7gXPpoo0</mediaplayer>
Calculation of linear regression[edit | edit source]
- Finally, using the equation of the calibration curve (equation or regression model) and the detected concentration, we calculate the theoretical absorbance value of the sample, which we compare with the absorbance measured by the instrument. We interpret the individual coefficients of the regression equation and evaluate the difference in absorbance.
- y = a + b * x Linear regression
- The formula for the general linear regression equation is based on Lambert-Beer's law. It contains two variables (x, y) and two regression coefficients (a, b). We can easily replace both variables with quantities from the graph of the calibration curve, which expresses the dependence of absorbance on concentration. Thus, x stands for concentration (c) and y for absorbance (A).
- A = a + b * c
- Now we just have to explain both regression coefficients. The regression coefficient a expresses a possible systematic measurement error (or deviations caused by dirt on the cuvette wall, etc.). By assuming its existence, we "filter out" these errors.
- The linear term b * calready corresponds to the "pure" Lambert-Beer law. According to him, the absorbance of the solution is equal to A = ε * l* c. The concentration appears in both expressions, therefore we can say that the regression coefficient b is equal to the product of the molar extinction coefficient and the width of the cuvette. This element tells us the direction of the straight line in the regression equation.
- b = ε * l .
- From there we determine the molar extinction coefficient ε = b / l .
- However, we must pay attention to the units, because the concentration is given in micromoles per liter (uMol/L), whereas the width of the cuvette is measured in centimeters.
References[edit | edit source]
Amler E. et al. Practical tasks from biophysics I. Institute of Biophysics UK, 2nd Faculty of Medicine, Prague 2006
Navrátil L. et al. Medical biophysics, Grada Prague 2005
On-line educational hypermedia – Hypermedia for Science Education) – http://elchem.kaist.ac.kr/vt/index.htm
UV VIS Spectrophotometers SPECORD® 50, SPECORD® 40, SPECORD® 30 with built-in control. Operating manual. Analyst Jena AG. ChromSpec cf. Publication Number: 221:408.23, Issued - February 2002
WinASPECT®. User Manual
Wikiscript of the 2nd Faculty of Medicine, Charles University in Prague https://www.wikiskripta.eu/w/Spektrofotometrie_(2._LF_UK) (21.10.2017)
ROSINA AND THE COLLECTIVE RETURNED., Medical Biophysics, Ed. 1. Prague : Grada, 2005. 524 pp. :, ill., portraits, tables. Avicenum. ISBN 80-247-1152-4.
FREEDOM AND THE COLLECTIVE., '"Review of secondary school physics," 1st ed. Prague: State Pedagogical Publishing House, 1991. 588 pp., illus. Cube. ISBN 80-04-22435-0.
KVASNICOVA, BÁLÍNOVÁ.Determination of creatinine concentration in urine [online]., [cit.2015-11-26]. Available from: <http://old.lf3.cuni.cz/chemie/cesky/praktika/uloha_B1.htm>