Spectrophotometry
A number of determinations in biochemistry make use of the fact that many substances absorb electromagnetic radiation in the visible or ultraviolet part of the spectrum, less often infrared radiation . The extent to which a substance absorbs light of different wavelengths (ie, the absorption spectrum) depends on the structure of the compound. The amount of light of a certain wavelength that is absorbed by, for example, a substance dissolved in a solution depends on the concentration of the substance . The measurement of light absorption by a sample is among the most widely used techniques in biochemistry and is referred to as photometry (when measured at one or more specific wavelengths) and spectrophotometry(if measured over a certain continuous range of light wavelengths).
Colour of fabrics[edit | edit source]
A number of substances contain a valence electron that can be excited to a higher energy level by electromagnetic radiation. Such a substance then absorbs radiation of a certain wavelength with photon energy corresponding to the energy difference of the two electron levels. If the absorbed radiation lies in the visible part of the spectrum , the substance will appear colored to the human eye (it will have a color complementary to the color of the absorbed light).
| Absorbed wavelenght (nm) | Matching colour | Complementary colour | ||
|---|---|---|---|---|
| 380–435 | purple | yellow-green | ||
| 435–480 | blue | yellow | ||
| 480–490 | greenish blue | orange | ||
| 490–500 | teal | red | ||
| 500–560 | green | magenta | ||
| 560–580 | yellow-green | purple | ||
| 580–595 | yellow | blue | ||
| 595–650 | orange | greenish blue | ||
| 650–760 | red | teal |
Unfortunately, it is not easy to predict the color of a substance based on its chemical structure, nor is it possible to unambiguously infer the composition of the substance based on the absorption spectrum. However, from our point of view, three groups of substances that are often colored are significant:
- Substances containing a system of conjugated double bonds , the molecule of which is not symmetrical. If we imagine a symmetric conjugated double bond system, it can exist in two resonance states that are energetically equivalent:
- The presence of an asymmetric substituent causes the energies of the two states to differ. The energy difference often corresponds to the energy of a photon from the visible part of the spectrum. Typical representatives can be dyes with a polymethine chain (–CH=CH–CH=CH–) or azo dyes (–N=N–). Substances with aromatic or heterocyclic structures bound to a common central atom (e.g. triphenylmethane dyes) behave similarly.
- Also, the d and f valence electrons often determine the color of the compound. They tend to be present in coordination covalent bonds of complex compounds . For example, anhydrous copper sulfate CuSO4 is colorless, while its pentahydrate CuSO4•5H2O and its aqueous solution are blue: in both cases, copper enters a complex with water [Cu(H2O)4 ]2+. In a similar way, colored and complex compounds of other transition metals (Fe, Cu, Cr, Mn, Ni, Co) tend to be complex-bound metal also in the colored proteins of hemoglobin and cytochromes.
- Ions that contain a transition metal with a high oxidation number as a central atom, e.g. MnO4-, Cr2O7 2-, are also colored.
Analytical methods used in medicinal chemistry and biochemistry make use of all three groups of colored compounds. Systems of conjugated double bonds are often formed in reactions in which the analyte condenses with a suitable chromogen (e.g. creatinine with picric acid in the Jaffé reaction, diazo coupling reactions in the detection of bilirubin), or are formed by oxidation of a chromogen that contains one less double bond (oxidation of benzidine derivatives in peroxidase reactions). The formation of colored complexes is used, for example, in the determination of proteins by the so-called biuret reaction (Cu2+ complexes with O and N peptide bonds) or in the detection of a number of substances, e.g. with FeCl3. Color changes during the reduction of Cr6+ to Cr3+ is used, for example, in the detection of ethanol in exhaled air.
Absorption of monochromatic light can also be conditioned by events other than electron excitation. These are primarily changes in the various oscillation energies of atoms in molecules and the rotational energies of entire molecules. These principles are used more in fluorimetry. From the point of view of medical biochemistry, they are much less important than the above principles.
Basic quantities and relationships used in spectrophotometry[edit | edit source]
Transmittance[edit | edit source]
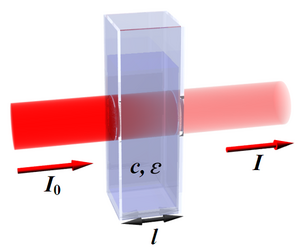
The amount of light of a certain wavelength that passed through the sample is described by the transmittance (lat. transmitto , I convert, let through). It is defined:
where
- T is the transmittance,
- I is the intensity of light that passed through the sample,
- I0 is the intensity of the light that entered the sample.
In practice, it would be inappropriate to measure exactly both intensities: in addition to the properties of the sample, they are also affected by the absorption and reflection of light on the walls of the cuvette and in the optics of the photometer , the environment in which the measurement takes place, etc. Therefore, the transmittance is usually measured relative to a blank sample. First, the intensity of light passing through a blank sample (blank, reference sample), i.e. a solution containing all components except for the determined color substance, is measured. Then, under the same conditions, the intensity of light passing through the unknown sample is measured. The transmittance is then defined by the relation.
where
- T is the transmittance,
- Iv is the intensity of light that passed through the sample,
- Ib is the intensity of the light that passed through the blank.
If the transmittance is measured in this way, there is no need to deal with non-specific losses of light intensity. The intensity of light that passes through the blank sample is considered to be 100% (ie, the transmittance of the blank is 100%), and the transmittance of samples absorbing light of a given wavelength is always less than 100%.
The transmittance of a solution that contains a colored substance depends on
- properties of absorbing substances,
- the wavelength of the transmitted light,
- amount of absorbing substance, i.e. on its concentration in the solution and on the thickness of the cuvette .
August Beer (1825–1863) formulated the dependence of transmittance on these quantities mathematically for the first time. Provided monochromatic light is used, it does
where
- T is the transmittance,
- ε is the molar decadal absorption coefficient (a constant specific for a given substance at a certain wavelength),
- l is the optical length of the cuvette ,
- c is the substance concentration of the absorbing substance.
We can also express the transmittance by algebraic adjustments
nebo ,
on the basis of which absorbance and optical density are defined
Absorbance[edit | edit source]
Absorbance is a quantity used in photometry and spectrophotometry . It indicates how much light was absorbed by the measured sample. In the literature, we can also encounter the older term extinction E .
We can define absorbance based on transmittance as:
- A = –log T,
- where:
- A is the absorbance;
- T is the transmittance of the same sample under the same conditions.
- From the definition of transmittance , the relations for absorbance follow:
The last relationship is referred to as the Lambert-Beer law (Johann Heinrich Lambert, 1728–1777). The practical advantage of its use is that the absorbance is directly proportional to the concentration of the absorbing substance.
From the equations above, it is clear that a sample that absorbs no light will have zero absorbance, an absorbance of 1 means that exactly one-tenth of the light has passed through the sample, with an absorbance of 2 exactly one-hundredth of the incoming light, etc. A negative absorbance would mean that more is passing through the sample light than a blank, usually due to a gross error or incorrect design of the experiment.
Absorbance is a dimensionless quantity.
Spectrophotometer[edit | edit source]
Photometers and spectrophotometers are used to measure quantities such as absorbance and transmittance , but sometimes, for example, also turbidance . Devices that measure at one or only a few precisely defined wavelengths of monochromatic light are called photometers . Technically more complex and perfect devices that allow the wavelength of monochromatic light to be set arbitrarily, or to measure a part of the absorption spectrum in a certain section of wavelengths, are called spectrophotometers.
Photometer arrangement[edit | edit source]
In principle, both the photometer and the spectrophotometer consist of four parts:
- light source
- monochromator
- the compartment in which the sample is placed
- detector
Light source[edit | edit source]
A suitable light bulb or discharge lamp serves as a light source . Incandescent and halogen lamps provide radiation with a continuous spectrum in the visible and infrared regions , but cannot be used for measurements in the UV region . Hydrogen or deuterium discharge lamps are most often used as sources of ultraviolet radiation . The source of UV and visible light can also be, for example, a xenon discharge lamp , but the wide range of wavelengths is balanced by some disadvantages: its light is a composition of a continuous and line spectrum, so there are large differences between the intensities at different wavelengths, the discharge lamp is very expensive and its intensity the light is not very stable.
Monochromator[edit | edit source]
Polychromatic light then passes through a monochromator. The simplest and cheapest option is to include a suitable interference filter in the optical path. Filters for practically any wavelength in the ultraviolet and visible regions are commercially available today. There are several types of filters, the appropriate combination of which creates a filter with the required properties. Low-pass filters pass light of wavelengths shorter than a certain limit (cut-off). High-pass filters, on the other hand, only let in light that has a longer wavelength than the cutoff wavelength of the filter. Bandpass filters pass a certain range of wavelengths. Since the boundaries are not always sharp, the lower and upper limits are usually the wavelength for which the filter has a 50% transmittance compared to the wavelength it transmits best. Sometimes the center wavelength that the filter passes and the bandwidth (or half-bandwidth) are also given.
Currently, an optical grating is usually used as a monochromator , by tilting which the wavelength can be continuously changed (e.g. the so-called Czerného-Turner monochromator). The range of wavelengths that emerge from the monochromator is determined by the slit, either fixed or also adjustable. The wider the slit, the greater the intensity of the outgoing light, but at the cost of less measurement specificity. On the other hand, a narrower slit will ensure more accurate adherence to the required wavelength, but at the cost of lower light intensity and deterioration of the signal-to-noise ratio.
Sample[edit | edit source]
Monochromatic light passes through the sample. We mostly work with solutions that are filled into standard cuvettes with an optical path of 1 cm. The cuvettes are placed in the cuvette in the instrument, which ensures their exact position, can be tempered and sometimes also contains a magnetic stirrer, which can be used to mix the contents of the cuvette after inserting the stirrer into the cuvette during the measurement. It is often possible to insert several cuvettes into the cuvette at the same time, which are then automatically inserted into the optical path.
Detector[edit | edit source]
The light emerging from the sample finally hits a detector, usually a photodiode or other photoelectric element. The intensity is evaluated using a system of transducers, compared to the intensity of light passing through the blank, and thus the absorbance is obtained . The accuracy of the measurement affects the integration time– the time for which the absorbance is measured. The longer it is, the more accurate the measurement result will be, unless, of course, the absorbing substance is photosensitive (ie, if the sample does not fade with longer exposure). The disadvantage of a long integration time is of course also the extension of the measurement time, which is essential especially when processing a large number of samples, when measuring at a large number of wavelengths (i.e. when measuring spectra), or when processing samples that change over time (kinetic measurements) .
In addition to the so-called single-beam photometers, in which a blank sample is first measured and then the measured sample is inserted into the same optical path, so-called double- beam photometers are also used , which are equipped with two detectors and allow the blank and the measured sample to be measured simultaneously in two optical paths.
Other spectrophotometer arrangements[edit | edit source]
Measuring a spectrum on an optical grating spectrophotometer means that the instrument measures the absorbance at one wavelength , then moves the grating, measures at another wavelength, and this is repeated continuously until the entire desired area is measured. This entails a relatively long measurement time, which can be a drawback for several reasons.
- the sample can change over time (especially in kinetic measurements)
- the sample may be photosensitive, it fades during the measurement
- if multiple samples are processed that are not sufficiently stable, it may be technically difficult to ensure the same conditions for the first and last samples
- the measurement takes a long time, the performance of the method is low
Diode array[edit | edit source]
The aforementioned shortcomings are eliminated by another arrangement of the spectrophotometer, measurement using a diode array (diode-array). With it, white light passes through the sample, which is then split into individual wavelengths (usually using a fixed optical grid ) and falls on a plate with a large number of detectors - photodiodes(hence the name diode array). The diode array is positioned in such a way that a certain (relatively narrow – e.g. 2 nm) range of wavelengths falls on each photodiode. The device does not contain any moving elements, which increases the reproducibility of the measurement, and in addition, the entire spectrum is measured at once. The measurement time can thus be reduced from several minutes to fractions of a second. In addition, this arrangement makes it possible to construct devices approximately an order of magnitude more accurate than classic photometers, which also require practically no maintenance, calibration, etc. The fundamental disadvantage is the purchase price is many times higher, but with the reduction in the price of miniature electronic elements, the cost of producing a diode array decreases rapidly.
Vertical beam photometry[edit | edit source]
In routine use, the classic arrangement of photometry also has other disadvantages:
- requires a large amount of sample
- it has low performance, the processing of individual samples is laborious
- cuvettes are expensive and difficult to maintain
- spectrophotometers are expensive
These disadvantages are largely eliminated by measuring in microtitre plates using a photometer with a vertical beam (plate reader). Samples are filled into polystyrene plates with 96 wells, but there are also other formats (from 4 to 384 wells). Special aids are available for working with microtitre plates (multichannel pipettes , repeater pipettes, etc.) that significantly speed up sample preparation. Unlike classical photometers, in which the absorbance is measured by a horizontal beam and the optical path is given by the thickness of the cuvette, in this case the measurement takes place using a vertical beam and the length of the optical path depends on the height of the level in the well. If we add a certain amount of colorless solution to the sample, the concentration of the absorbing substance will decrease, but at the same time the level of the solution in the well will increase proportionally (the optical path will be extended) and the resulting absorbance will be the same. Conversely, if part of the colorless solvent evaporates from the sample, the optical path will be shortened, but the concentration of the absorbing substance will rise and the absorbance will not change again. It can be said that, unlike classical photometry, where the absorbance corresponds to the concentration of the absorbing substance in the solution, the absorbance when measured with a vertical beam depends on the quantity of the absorbing substance in the sample. For vertical beam photometry, a much smaller volume of sample is required – the wells are usually filled with only 100 to 300 μl of solution. Microtitre plates are disposable and, given the number of samples measured at the same time, are significantly cheaper than plastic cuvettes. Measuring all 96 wells usually takes only a few seconds. Also, plate photometers tend to be relatively cheap, as a monochromator they usually use a set of interference filters. Vertical beam photometry is especially often used inimmunochemistry , especially in the ELISA methodology (hence the extended designation of the device ELISA-reader). For greater clarity, vertical beam photometry uses the term optical density (OD) in addition to the term absorbance. At the same time, the relationship applies
- OD = A / l
- where OD is the optical density , A is the absorbance and al is the optical path length.
The optical density therefore depends only on the properties of the sample, but not on the length of the optical path along which the light passes through the sample.
Reflectance photometry[edit | edit source]
Another photometric technique is reflection photometry – a technique routinely used, for example, in clinical biochemistry within so-called dry chemistry. In dry chemistry, biological material is not processed in the usual way in test tubes or other containers, but is applied to a film impregnated with individual components of the reaction mixture. The result is a change in the color of the field, which can be quantified using a reflectance photometer - the loss of light of a certain wavelength reflected from the field is measured. Either LED is used as a light sourcediodes emitting a suitable wavelength, or light sources with interference filters. To increase the sensitivity, the reflected light is focused on the detector by a convex mirror (the so-called Ulbricht sphere). A classic example of dry chemistry and reflection photometry is the examination of urine using diagnostic strips, however, due to its simplicity and speed with an accuracy comparable to classical methods, the number of applications is rapidly expanding.
Advanced spectrophotometric techniques[edit | edit source]
Multicomponent analysis[edit | edit source]
It often happens that in the part of the spectrum where the substance to be determined has an absorption maximum, another component of the reaction mixture also absorbs at the same time. In that case, it is not easy to determine the concentration of the measured substance, because the absorbance at the selected wavelength is the sum of the absorbances of both substances. If the concentration of the second, "interfering" component is known, or if the experiment can be set up so that it is constant in all samples, this situation can be resolved by using suitable blanks. In some cases, however, it is necessary to proceed with the so-called multicomponent spectrum analysis methods .
It is usually not measured at a single wavelength, but rather a continuous part of the spectrum is measured, or at least measured at several wavelengths. If the extinction coefficients of individual substances absorbing in a given region at different wavelengths are known (or even better, if the absorption spectra of individual components of the mixture are known), the concentration of the analyte can be calculated by solving a system of equations.
In the simplest case, it is necessary to determine the concentration of one substance, while its absorption spectrum (dotted in the figure) overlaps the spectrum of another substance present in the sample (dashed). By measuring the sample, we obtain a spectrum that is the sum of both absorption spectra (solid line). Even if we know that the measured substance has an absorption maximum at the wavelength λ1 , we cannot determine its concentration directly, because at this wavelength the absorbance of the other substance cannot be neglected. However, if we know the shape of the absorption spectrum of the second substance, we can find the wavelength at which it absorbs the same as at the wavelength λ1 (let's call it λ2), i.e. the extinction coefficient is the same for λ1 and λ2. Then the absorbance of the determined substance itself will be
- A = A(λ1) – A(λ2).
Using the isosbestic point[edit | edit source]
If a substance that has a certain spectrum changes during some reaction to another substance that has a different spectrum, but the two spectra partially overlap. An example can be the spectra of NAD+ and NADH (the determination of these coenzymes is used when measuring the activity of a number of enzymes using the so-called Warburg optical test ). In this case, the absorption maxima are far enough apart that the determination of the individual forms of the coenzyme is simple. However, the fact that all the spectra for different NAD+ /NADPH ratios (at constant total concentration) cross over may be useful. This is because at a wavelength of 281 nm it has NAD+ and NADH the same extinction coefficient. The intersection of the spectra is called the isosbestic point (from the Greek ισος isos = the same and σβεννυμι sbennými = I extinguish) and by measuring the absorbance at this point we can easily determine the total concentration of NAD+ and NADH without needing to know the actual concentration ratio of the two components.
Other methods[edit | edit source]
A number of other data can be obtained by appropriate mathematical processing of the measured spectra. When measuring more complex systems, derivative spectrophotometry can help , which enables, after evaluating the first and second derivatives of the spectra according to wavelength, for example to find the exact positions of absorption maxima, etc. Mathematical processing of the obtained data is also necessary for the processing of kinetic measurements.
Accuracy of photometric methods[edit | edit source]
Most photometers can measure absorbance in the range of 0 to 3 or 4. However, this does not mean that measuring over this entire range is reasonable. Assuming that the random error of the detector will always have the same properties, the average relative error of the absorbance measurement as a function of the actual absorbance of the sample will have a U-shaped curve. The best results will be obtained if the absorbance of the sample is in the range of 0.2 to 0.8, measurements in the range of 0.2 to 1.2 can be considered reasonable. If the absorbance is higher, it is advisable to dilute the sample. Of course, the specific values depend on the properties of the photometer used, but the course of the average relative error as a function of absorbance will still be the same (only the "stretching" of the curve differs).
The accuracy of the measurement is also greatly influenced by the preparation of the sample and cuvette . Let us list at least the most significant influences that affect the accuracy of spectrophotometric determinations:
- must be measured in a suitable cuvette, the selected wavelength must lie in the band for which the cuvette is intended
- if measured in multiple cuvettes, they should all have the same factor. The plastic cuvettes should all be from the same batch.
- cuvettes for samples and blanks must be clean. This can be verified by filling them with distilled water all giving the same absorbance.
- the cuvettes must be handled in such a way as not to contaminate the optical surfaces (e.g. by touching the fingers). The same applies to microtitre plates.
- the cuvette must be dry on the outside, no air bubbles must remain inside, the measured solution must be homogeneous. A drop running down the cuvette, bubbles or a floating precipitate in the measured solution will usually show that the measured absorbance is constantly changing.
- the cuvette must be sufficiently filled with the sample.
- if several samples are measured successively in one cuvette, it is necessary to work in such a way that the error caused by the residues of the previous solution is as small as possible. Usually the cuvette is rinsed with distilled water between samples and then dried as best as possible. More accurate results can be obtained if, after washing, the cuvette is rinsed with a small amount of sample, which is poured out and only then is the cuvette filled with the required amount of sample for measurement. If working with several similar solutions, it may be more accurate not to rinse the cuvettes with distilled water between them, just pour them out and dry them as best as possible.
- when measuring in cuvettes, the concentration of the colored substance in the solution is essential, therefore, when preparing the sample, it is most important to observe the ratio of the individual components. It is advisable to arrange the experiment in such a way that all components are pipetted with the same pipette, preferably fixed.
- If it is measured in microtitre plates, on the other hand, the substance amount of the colored substance in the sample is decisive. Therefore, the experiment must be organized in such a way that the analyte is measured into the well as precisely as possible. A large error can also be caused by the different shape of the surface in the individual wells, therefore all samples should have the same affinity to the walls of the plate, or it is advisable to mix the plate briefly before the measurement to ensure that the walls are wetted even above the solution level.
Cuvette[edit | edit source]
A cuvette is a laboratory aid for measuring the optical properties of solutions. Standard cuvettes with an optical path of 1 cm are most often used (rarely shorter – so-called ultra-microcuvettes for very small volumes of measured solutions). Cuvettes can be made of different materials and have different designs.
Cuvette material[edit | edit source]
Cuvettes made of optical glass (usually labeled OG = optical glass, G = glass, etc.) are suitable for measurements in the visible part of the spectrum . For measurements in the UV range , a cuvette made of quartz glass (Q = quartz, UV) must be used. Cuvettes made of special optical glasses (eg OS = Optisches Glas) are also available, which can usually be used for a wider part of the spectrum than conventional optical glass cuvettes, but are cheaper than quartz cuvettes. Measurement in cuvettes made of different types of glass is very accurate, with the right technique absorbance can be determinedsample with accuracy up to four or five decimal places. However, the cuvettes are relatively expensive - optical glass costs hundreds to thousands of crowns, while the price of a normal quartz glass cuvette is around four to five thousand crowns. At the same time, the service life of cuvettes is limited, moreover, their maintenance is quite laborious. For these reasons, disposable plastic cuvettes are used for routine measurements, the price of which is usually only a few crowns, on the other hand, they can be reliably measured with an accuracy of only two to three decimal places (which is, however, fully satisfactory for most applications). They are mostly made from polystyrene (PS) - for the visible part of the spectrum, or from polymethyl methacrylate (PMMA) - also for part of the UV range.
Cuvette dimensions[edit | edit source]
Today, standard spectrophotometric cuvettes (so-called macrocuvettes ) have internal dimensions of 1×1×3 to 4 cm and are filled to a volume of 3 ml (or less depending on the arrangement of the photometer and the height of the beam above the bottom of the cuvette). Due to the fact that we are working with smaller and smaller samples, semi-microcuvettes are used more and more often , which have a narrowed sample space and only need to fill them with about 0.8 ml of sample (again, it depends on the design of the device). Micro- and ultra-micro cuvettes are also available, which (sometimes at the cost of shortening the optical length) can also work with significantly smaller volumes (on the order of microliters). Because when semi-micro-, micro- and ultra-micro cuvettes are used in some spectrophotometers, a significant part of the light would pass through the glass around the sample, which would significantly increase the background and worsen the measurement accuracy, these cuvettes are often so-called masked – the glass around the sample area is blackened. On some cuvettes, the so-called cuvette factor is indicated - actually the actual optical length of the cuvette in cm. Ideally, the cuvette factor is equal to one, but it can vary due to manufacturing inaccuracies.
Special cuvettes[edit | edit source]
Other types of cuvettes are used for special applications, e.g. flow cuvettes that can be connected to e.g. chromatography instruments, tempered cuvettes, spectrophotometric capillaries, etc.