Radiotherapy
(Redirected from RADIOTHERAPY)
Introduction[edit | edit source]
Although radiation is known by scientists only since 1890s, there exists a wide variety of uses in the medical field. We can use it to diagnose or monitor the medical condition of the pacient. The most common procedure is taking an x-ray to diagnoze a bone fracture, since the radiation is absorbed by hard tissues such as bones. Other common diagnostic procedure is computer tomography (CT), which functions the same way as a x-ray, but the measurements are taken from different angles, which forms a 3D picture. We can also use radiation in oncology to kill or control malignant cells. This process is called radiotherapy. The ionizing radiation used during this treatment can be classified as electro-magnetic radiation or corpuscular radiaton. The negative side effects are due to the part of the radiation, which is absorbed by the tissues, not by the one, which passes through.
Biological mechanism of the effect of the ionizing radiation[edit | edit source]
Radiation in general functions by damaging the DNA of the cell. This DNA damage is caused by one of two types of energy, photon or charged particle. The effects of ionizing radiation can be diveded in direct or indirect radiation of the tissue. Both of them lead to excitation of an atom or molecule. This process consists of increasing the energy in the atom and bringing it to a higher energy level, i.e. getting further from the nucleus. During ionizition the electron gets away from the atom and changes to an ion.
Indirect ionization occurs as a result of the ionization of water, forming free radicals (H• and OH•), which then damage the DNA by interacting with its free molecules. Leading to DNA breakage, which can cause mutations in the radiated cell.
Direct ionization occurs if the energy of the radiation is absorbed directly in the nucleus.
The biological effect hangs on the dosage of the radiation, which constits of the total amount of energy absorbed by the radiated tissue. Because cells have the ability to repair DNA damage with their enzymes, we have to limit the amount of time given to these reparations. This leads to the need of dose fractionation, because we do not want the healthy cell to be demaged, but neither do we want the malignant cell to grow.
Beside the radicals formed during indirect radiotherapy, other products are made. To the production of new molecules, oxygen is very important, which means the cells contaning less of it are less radiosensitive. The presence of oxygen inhibits the reparations occuring in the DNA and increases the amount of radicals in the cell.
Cells have also mechanisms to repair single-strand DNA damage, and therefore double-stranded DNA breaks prove to be the most efficient technique to cause cell death. Cancer cells are generally undifferentiated and stem cell-like; they reproduce more than most healthy differentiated cells and have a diminished ability to repair sub-lethal damage. Single-strand DNA damage is then passed on through cell division resulting in accumulation of DNA damage which cause them to die or reproduce at a slower rate. Therefor cells at the beginnig of their evolution, which are reproducing faster (bone marrow, intestinal epithelium), are more radiosensitive, than fully differentiated cells (brain, myocardium).
Biophysical basics of radiotherapy[edit | edit source]
The properties of ionising radiation are rectilinear spread decreases to the square of distance from the source. It will decrease upon passing through an absorbent medium due to interactions between the ionising radiation and the medium. The decrease in radiation is dependent on the type and energy of the ionising radiation and on the absorbent properties of the medium. The most significant losses are observed for heavy, charged particles and the least significant losses for photon radiation. When energy increases the radiation loss decreases but with increasing atom number and specific density of the absorbent medium the losses increase as well.
Ionising radiation can only cause measurable biological effects when the emitted radiation possesses high levels of energy. Ion pairs can be created by direct interaction of irradiating particles and electrons of the electron field (alpha particles, protons, deuterons and other charged particles) or by indirect interaction. This occurs by using a mediator in the form of charged particles, released by the interaction of gamma radiation, X-rays and neutrons.
The relationship between physical exposure and biological effects is basically directly proportional: the longer the exposition, the more intensive the expected biological effect. Other influencing factors are time, spatial distribution and quality of the radiation.
A single exposure describes a situation where the radiation is delivered during a short period of time (minutes). Fractionated exposure refers to when the total exposure is divided into fractions delivered over a period of several weeks.
The biological effects increase proportional to the volume of the irradiated tissue. Different qualities of radiation will have different ionising abilities per unit of track, and therefore different biological effects. The types of radiation with the highest values of linear energy transfer (i.e. heavy particles) have the most harmful biological effects.
Units[edit | edit source]
Absorbed dosage of radiation[edit | edit source]
Absorbed dosage (D) is defined by ratio of the energy of ionizing radiation (E) of the mass (m) of the object, which absorbed the radiation.
D = E/m
The unit of the absobed dosage is one Gray (Gy). It is used only for direct ionization.
[D] = J/kg = Gy
Kerma[edit | edit source]
Kerma (Kinetic energy released in material) describes the influence of secundary particles on the material. It’s unit is 1 Gray.
Equivalent dose[edit | edit source]
Every radiation has it’s own biological impact and demages the tissue in many different ways. The dosage equivalent (H) helps us to compare the impact of the radiation on our organism.
H =D × Q
[H] = J/kg = Sv
The unit describing the dosage equivalent is called 1 Sievert (Sv).
Effective dose[edit | edit source]
It is the addition of equivalent doses to all organs, each adjusted to account for the sensitivity of the organ to radiation.
The unit is 1 Sievert.
Fundamental principles of radiotherapy[edit | edit source]
When using ionising radiation for therapeutic purposes, it is always necessary to remember the radiobiological facts. Every dose of ionising radiation delivered to the human organism possess a certain strain which is accumulated over the course of the radiation cycles.
The main physical factor influencing the extent of biological effects of radiation is the absorbed dose (in Gy) within the tumour mass. The radiation procedure needs to be chosen in such a way, that the whole tumour is targeted homogenously with maximal dose. Meanwhile the surrounding healthy tissues receive as low dose as possible. The effect of given dose can differ based on the source of radiation and its linear energy transfer. Which is why the term relative biological effectiveness (RBE) was introduced. RBE is the ratio of standard ionisation dose to radiation dose with different linear energy transfer, which causes the same biological effect.
There are not only destructive and reparative processes inside the cells following the exposure to ionising radiation, but also proliferative processes take place within the tissues. This process helps to replace the destroyed and disintegrated cells. Different tissues show different speed of these proliferative processes.
The biological effects of radiation depend not only on the dose, but also on time. Therefore, in radio therapeutic practise the tumours are irradiated not only by a single dose, but in a fractionated way. The same dose is divided into several partial doses and applied in precisely set time intervals. This makes the final dose of radiation significantly lower. This phenomenon is explained by the ability of the tissue to recover from the exposure of radiation (so called recovery factor).
The method of fractionated exposure is based on dividing the total needed dose into a series of partial doses. They are applied daily or in other regular time intervals (so called classic fractionation is the application of 2 Gy daily in the target volume, 5 fractions a week result in a total amount of 20-30 fractions, total dose of 40-60 Gy). All other fractionation regimes are usually related to biological dose equivalents in classic fractionation. There are several different types of fractionation. For example hypo fractionation regime (irradiating with less than 5 fractions a week) and hyper fractionation regime, which uses the method of irradiating with several fractions a day (2 to 3). Tissues characterised by fast proliferation and regeneration are usually more tolerant to hypo fractionation, while tissues with slow proliferation, reparation and regeneration show higher tolerance to hyper fractionation.
The method of fractionation is always carefully chosen so that the suitable daily dose, total dose and number of fractions, along with the total number of days over which the dose is administrated, all lead to complete destruction of the tumour mass. But it must not exceed the tolerance of healthy tissues. The ratio between lethal tumour dose and dose tolerated by healthy tissues is called therapeutic ratio (also known as therapeutic index). If this ratio is lower than 1, the situation is very convenient for radiotherapy because the radiation dose can be applied to the tumour without any significant damage to the surrounding healthy tissues. If the ratio equals 1, the sensitivity of the tumour is limited. With continuous treatment there is a risk of a small percentage of complications. However if the ratio is higher than 1, the situation is not well suitable for radiotherapy. There are several ways of improving the effect of radiation on the tumour cell and thus increasing the therapeutic ratio: Increased supply of oxygen to the tumour – combination of radiation and hyperbaric oxygen.
Radio sensitizer are substances showing a significant oxygen effect and increase the amount of free radicals within the target volume which interfere with the reparative processes. The use of radiation with high linear energy transfer (LET) – for example when using a beam of fast neutrons, the effect is independent on the presence of oxygen. Neutrons represent highly ionising particles, which cause direct lethal damage to the tumour cells.
Combination of radiotherapy and cytostatics has addative effect. The lethal effect of both the modalities is combined at the same sensitive point of the cell cycle.
Methodology of radiation[edit | edit source]
Methodology of radiotherapy is very difficult and it requires a complex approach. The process begins with determining the exact location of a tumour and calculating the target volume. The next step is the preparation of isodose plan i.e. determining the size of the spatial dose distribution, integral dose, dose delivered to the target organ and to other distinctive points. Also specification of the length of the therapy etc. All the important parameters characterising one specific radiotherapy plan, needs to be transformed into irradiation protocol in such a way that the setting determined for each field would be reproducible over the whole course of the fractionated exposure.
There can be either systematic or random mistakes made during the course of radiotherapy. Systematic mistakes are made during the planning of radiotherapy and they are caused by incorrect determination of the target volume and incorrect calculation of the isodose plan. Random mistakes are related only to the individual fractions and are caused by incorrect setting of certain parameters. The extent of random mistakes does not have any significant effect on the overall result of the treatment.
Clinical problems of radiotherapy[edit | edit source]
Radiotherapy is one of the most effective tools used in the treatment of malignant tumours. However it needs to be used with consideration and all available medical and physical knowledge always have to be taken into account. It is important to realize that in some cases radiotherapy can be strictly contraindicated. These cases include very advanced malignancies, cachectic patients, disintegrated tumour, anaemia, febrile states etc.
Radiotherapists look very carefully for early reactions originating over the course of irradiation. These reactions are mainly skin related. In case of irradiating tumours located inside the body there is usually reactive changes found on the mucosa of exposed locations. Large doses of radiation cause systemic reaction (decrease in the blood formation).
Patients with malignant tumours undergoing radiotherapy are being regularly observed at the radio therapeutic clinics, so that even late post-exposure changes can be monitored. For adults, the most common reactions are permanent damage to hair follicles, skin glands also skin atrophy and trophic changes of the irradiated mucosa. Significant breach of the tissue tolerance (which should not occur when the radiotherapy is correctly planned) can lead to chronic changes such as pulmonary fibrosis, damage to the spinal cord and changes in the hepatic and renal parenchyma. There is a significant reduction of the vascular network due to the limited tolerance of the vascular wall.
Child patients require long-term observation following the completion of radiotherapy. This is due to the fact that their healthy, developing tissues are very radiosensitive. Examples of typical late effects of radiotherapy is stunted growth of long (and other) bones, changes in the teeth development, muscle hypoplasia, mammary gland hypoplasia or even aplasia etc.
All of the above mentioned side effects of radiotherapy can be classified as somatic post radiation changes. Additional to these there can also be genetic and oncogenic effects of radiotherapy. Genetic effects can affect not only the irradiated individual, but also children and grandchildren. The results of oncogenic effects can be the development of secondary „primary“ tumours, the origin of which was initiated by the ionising radiation intended to cure the „first primary tumour“.
Irradiators used in radiotherapy[edit | edit source]
Sources of gamma radiation used in radiotherapy usually employ radio nuclides emitting gamma radiation of suitable energy and convenient physical half-life.
Particle accelerator is a device used for artificial acceleration of electrically charged elementary particles or ions in order to provide them with enough kinetic energy (α and ß particles emitted by the radioactive atom’s nuclei possess rather low energy, and are therefore not suitable for radiotherapy) used in tumour irradiation. Accelerated particles can also be used in order to generate penetrative electromagnetic radiation.
Types of irradiators[edit | edit source]
Betatron is a type of cyclic particle akcelerátor. It can be used as a source of gamma or RTG radiation.
Linear particle accelerator is used for accelerating charged particles with the use of electrical field (electrostatic, induction or resonant). The accelerated particles are then rapidly stoped, which creates particles with high energy levels.
Cyclotron is a cyclic particle accelerator used for accelerating heavy charged particles (protons, deuterons, alpha particles and ions) along a spiral track. It is used in proton therapy
Basic types of radiotherapy[edit | edit source]
There are two main division of radiotherpy - external beam radiation therapy and brachytherapy. The main difference between these methods is the way of incorporating the radiation into the malignant tissue.
External beam radiation therapy[edit | edit source]
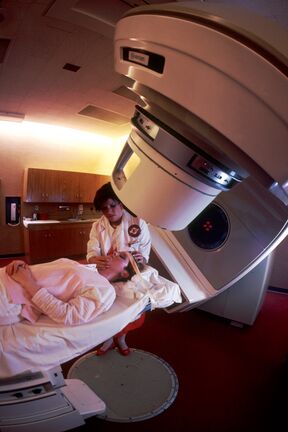
It is the most common kind of radiotherapy. The radiation is incorporated into the tissue from an external source (usually 80 or 100 cm from the body). The patient usually lays down and the ionizing radiation points toward a certain part of the body, where the cancerous cell are growing. We use the linear accelerator for this purpose.
Cobalt and caesium irradiators[edit | edit source]
Cobalt and caesium irradiators generate gamma radiation.
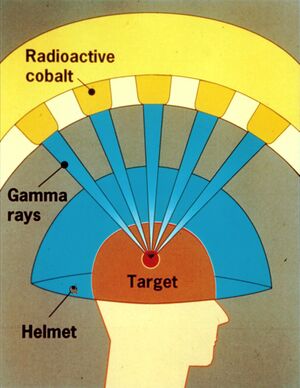
Cobalt irradiators belong among large irradiators, because they show high source activity (at least 3,7 . 1013 . s-1). They are usually used for deep radiotherapy. Radioactive cobalt 60Co (physical half-life of 5,29 years) emits two gamma radiation quanta characterised by energies reaching up to 1,33 and 1,17 MeV and high penetrability. 60Co is most commonly used in the form of flat rings or small rollers (1 x 1 mm) in an aluminium or steel container (24 x 24 mm). The protective cap is shaped like a sphere with diameter of up to 60 cm. The cap is made of lead and inside it contains a wolfram alloy/uranium core (both of them being more absorbent than lead). The cap has a channel-like opening through which it emits a primary beam of gamma radiation.
Radioactive Caesium 137Cs emits gamma radiation quantum with energy level of 0,66 MeV. Its physical half-life is 30,4 years. It is used for irradiating pathological malignancies located within the depth of 5 cm (maximum). Due to relatively long physical half-life of 60Co and 137Cs the intensity of gamma radiation produced by these irradiators decreases only very slowly over time.
Gamma knife[edit | edit source]
The scientist Lars Leksell was looking for options how to remove intracranial lesions atraumatically (without having to open the skull) with the help of ionising radiation. In 1951 he defined the principles of so called radio surgery. Radio surgery enables alteration of the pathological lesion after it has been precisely located by stereotactic methods. The basic principle itself is very simple. A sufficient amount of suitable ionising radiation sources are placed in such a way that the individual generated beams all intersect at a common focal point. In case of gamma knife, that means 201 sources of gamma radiation (cobalt isotope 60Co). While the dose delivered by individual sources (beams) is relatively small, the individual doses all add up at the common focal point, generating a very high radiation dose. This high dose causes appropriate biological response in the affected tissue – necrotic lesion. The dose delivered by one single beam would not cause any significant response. The radiobiological response of the tissue lasts from several days up to several years. The dose gradient outside the common focal point, where the beams intersect, sharply decreases within millimetre distances from the source. If the target we intend to hit during the functional stereotactic surgery is placed within the common focal point, a suitable radiation dose is able to generate small necrotic lesion in the pathologically changed tissue while the surrounding healthy tissues are untouched. The most common indication for radio surgery are arteriovenous malformations and both benign and malignant tumours (especially brain metastases).
This treatment shows zero mortality and very low morbidity. It eliminates certain risks of conventional radiotherapy, such as bleeding, infection and the need for postoperative convalescence. The patient can continue in his everyday routine the following day after the surgery.
Brachytherapy[edit | edit source]
The source of radiation is placed near the tumor or eventualy in the tumor itself. This enables us to employ more radiation in a shorter amount of time and not damage the healthy tissue around it. This kind of therapy is most commonly used in gynaecology and tumors of gastrointestinal tract.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- ZOUL, David. Biologické účinky ionizujícího záření, http://www.cytoprostor.euweb.cz/radiobiologie/radiobiologie.pdf
- Česká onkologická společnost České lékařské společnosti Jana Evangelisty Purkyně, https://www.linkos.cz/pacient-a-rodina/lecba/jak-se-lecit/radioterapie-ozarovani/
- MUDr. L. HYNKOVÁ, MUDr. H. DOLEŽALOVÁ, Ph.D., prof. MUDr. P.ŠLAMPA, CSc. Radioterapie. Učební texty pro studenty 5. roč. LF MU Brno Klinika radiační onkologie, LF MU.
- Základní princip gama nože, https://www.homolka.cz/cs-CZ/oddeleni/stereotakticka-a-radiacni-neurochirurgie-osrn/lekselluv-gama-nuz.html
- Dirk VeRELLEN, Mark De RIDDER, Nadine LINTHOUT, Koen TOURNEL, Guy SOETE & Guy STORME. Innovations in image-guided radiotherapy. Nature Reviews Cancer 7, 949 -960 (December 2007)
- NAVRÁTIL, Leoš a Jozef ROSINA. Medicínská biofyzika. Vyd. 1. Praha: Grada, 2005, 524 s. ISBN 80-247-1152-4.
References[edit | edit source]
- Radiotherapy - textbooks for students 5th year. LF MU Brno. Department of Radiation Oncology, Faculty of Medicine, Masaryk University. Prepared by: MUDr. L. Hynková, MD H. Doleželová, Ph.D., prof. MUDr. P. Slampa, CSc.
- ↑Jump up to:a b Society of Radiation Oncology, Biology and Physics, https://www.srobf.cz/en/home/
- ↑ Basic principle of gamma knives, https://www.homolka.cz/cs-CZ/oddeleni/stereotakticka-a-radiacni-neurochirurgie-osrn/lekselluv-gama-nuz.html
- ↑ Protonová léčbahttp://www.ptc.cz/protonova-lecba/princip-lecby/