Nonsense-mediated mRNA decay
Nonsense-mediated mRNA decay (NMD) is a pre-translational process that prevents the production of shortened proteins that would be sythesized due to the presence of a premature stop codon (so-called nonsense mutation) in the mRNA. Such defective mRNA is degraded.
Defense against defective mRNA[edit | edit source]
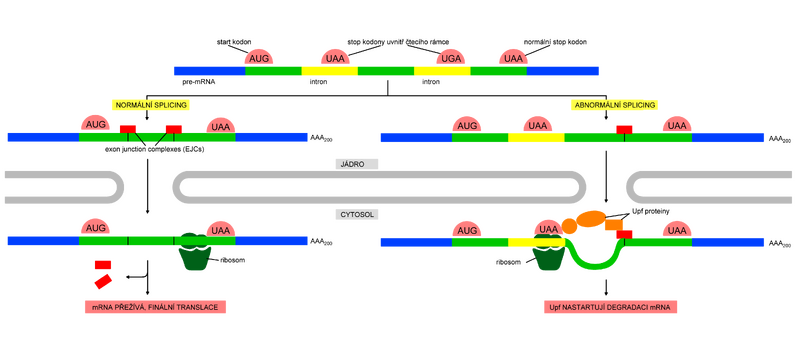
In eukaryotes, mRNA production includes not only its own transcription, but also a number of other modifications. These processes take place in the nucleus, i.e. independently of ribosomes. Only when post-transcriptional modifications are successfully completed, mRNA molecules are transported to the cytoplasm, where their fate is completed by translation on ribosomes. However this process is not completely error-free, and it can sometimes happen that "carelessly" edited mRNA is sent to the cytoplasm. In addition, even mRNA that originally left the nucleus properly edited can be damaged or disrupted in the cytosol. The danger of translation of damaged or incompletely edited mRNA is so high that the cell has several other backup mechanisms to prevent the resulting protein from being shortened or otherwise defective. In order to avoid translation of damaged mRNA, translation initiation mechanisms first of all recognize the 5' cap and the poly-A tail before its initiation. In order to ensure that the mRNA is spliced correctly before translation, a small group of proteins, the so-called exon junction complex (EJC), is bound to the junctions of exons during splicing, which then serve as an initial control (see below).
Abnormal splicing[edit | edit source]
The most important mRNA surveillance system, called "nonsense-mediated mRNA decay" (NMD), eliminates defective mRNA before it can be fully translated into protein. This mechanism is brought into play the moment the cell discovers that the mRNA molecule carries a nonsense (stop) codon (UAA, UAG, UGA) in the "wrong" place - a situation often caused by inaccurate splicing. Abnormal splicing often causes the random "inclusion" (or rather non-excision) of a nonsense codon in the mRNA reading frame. This happens especially in organisms such as humans where introns are enormous in size.
NMD mechanism[edit | edit source]
NMD begins as soon as the mRNA is transported from the nucleus to the cytosol. As soon as its 5' end emerges from the nuclear pore, it binds to the ribosome, which immediately begins translating the mRNA. As translation progresses, exon junction complexes (EJC), bound to the mRNA at the sites of each splice site, are progressively removed by the moving ribosome. Normally, the stop codon is at the end of the last exon, so by the time the ribosome reaches it and terminates translation, no EJC should be bound to the mRNA. However, if the ribosome finds a premature stop codon and thus premature termination occurs, it "senses" that there is still some EJC left and the bound mRNA is degraded. In this way, the first round of translation allows the fitness of any mRNA to leave the nucleus to be tested.
Meaning of NMD[edit | edit source]
The importance of NMD could be particularly applied in evolution, when it allowed eukaryotic cells to more easily "discover" new genes creted by modifications, mutations, or alternative splicing patterns - namely, by selecting for translation only those mRNAs that could produce full-lenght proteins.
NMD is also important for cells of the developing immune system, where extensive DNA rearrangements often result in premature termination codons. Indeed, NMD surveillance degrades the mRNA from such "rebuilt" genes, thus preventing the potential toxic effects of shortened proteins.
In addition, NMD plays a significant role in alleviating the symptoms of many inherited diseases. Hereditary diseases are usually caused by mutations that disrupt the function of a certain key protein, such as hemoglobin or factors of the coagulation cascade. About one-third of all genetic disorders in humans are caused by "nonsense mutations" or mutations (eg farmeshift mutations or splice site mutations) that result in the placement of a premature stop codon in frame. In individuals who carry one mutated and one functional gene, NMD eliminates the aberrant mRNA, thereby causing the toxic protein not to be produced at all. Without this safety net, people with one functional and one mutated "disease causing" gene would be more likely to show far worse symptoms.
Links[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
References[edit | edit source]
- ALBERTS, Bruce. Molecular Biology of the Cell. 5. edition. Garland Science, 2008. ISBN 978-0-8153-4106-2.
