MOLECULES & SOLUTIONS
Molecules and solutions are fundamental elements of intracellular and extracellular matrix of the human body. The most important biophysical features of molecules and solutions for processes in human body are the colligative properties of solutions, the osmosis and the surface tension. Properties of solutions differ from both properties of the solutes or of the solvent, and they can be divided into two main groups--colligative and non-colligative properties.
COLLIGATIVE PROPERTIES[edit | edit source]
Colligative properties depend only on the osmolarity, i.e. number of dissolved particles in solution and not on their chemical nature (types of solutes). Non-colligative properties depend on the identity of the dissolved species and the solvent.
Increase of solution osmolarity causes:
- Osmotic pressure increase
- Vapour pressure decrease
- Freezing point decrease
- Boiling point increase
They have following consequences:
- Solution with higher osmolarity stays as liquid over larger range of temperatures compare to solution with lower osmolarity.
- The reduction of vapour pressure of a solvent is proportional to the molar concentration of the solute (Raoult's law).
Raoult's law[edit | edit source]
Raoult’s law (1.1) describes the situation when a non-volatile solute is added to a liquid to form a solution, then the vapour pressure above that solution decreases. To understand why that might occur, let's analyse the vaporization process of the pure solvent then do the same for a solution.
Liquid molecules at the surface of a liquid can escape to the gas phase when they have a sufficient amount of energy to break free of the liquid's intermolecular forces. That vaporization process is reversible. Gaseous molecules coming into contact with the surface of a liquid can be trapped by intermolecular forces in the liquid. Eventually the rate of escape will equal the rate of capture to establish a constant, equilibrium vapour pressure above the pure liquid. If we add a non-volatile solute to that liquid, the amount of surface area available for the escaping solvent molecules is reduced because some of that area is occupied by solute particles. Therefore, the solvent molecules will have a lower probability to escape the solution than the pure solvent. That fact is reflected in the lower vapour pressure for a solution relative to the pure solvent.
Previous statement is only true if the solvent is non-volatile. If the solute has its own vapour pressure, then the vapour pressure of the solution may be greater than the vapour pressure of the solvent. Note that we did not need to identify the nature of the solvent or the solute (except for its lack of volatility) to derive that the vapour pressure should be lower for a solution relative to the pure solvent. That is what makes vapour pressure lowering a colligative property - it only depends on the number of dissolved solute particles.
Mathematical description of the vapour pressure lowering phenomenon was given by F.M. Raoult as
p = χsolvent·p0 (1.1)
Raoult's law (1.1) describes the mathematics of vapour pressure lowering. It states that the vapour pressure of a solution (p) equals the mole fraction of the solvent multiplied by the vapour pressure of the pure solvent (p0). If a pure solute which has zero vapour pressure (it will not evaporate) is dissolved in a solvent, the vapour pressure of the final solution will be lower than that of the pure solvent. While that "law" is approximately obeyed by most solutions, some show deviations from the expected behaviour.
Deviations from Raoult's law (1.1) can either be positive or negative. A positive deviation means that there is a higher than expected vapour pressure above the solution. A negative deviation, conversely, means that we find a lower than expected vapour pressure for the solution. The reason for the deviation stems from a flaw in our consideration of the vapour pressure lowering event - we assumed that the solute did not interact with the solvent at all. That, of course, is not true most of the time. If the solute is strongly held by the solvent, then the solution will show a negative deviation from Raoult's law (1.1) because the solvent will find it more difficult to escape from solution. If the solute and solvent are not as tightly bound to each other as they are to themselves, then the solution will show a positive deviation from Raoult's law (1.1) because the solvent molecules will find it easier to escape from solution into the gas phase.
Solutions that obey Raoult's law are called ideal solutions because they behave exactly as we would predict. Solutions that show a deviation from Raoult's law are called non-ideal solutions because they deviate from the expected behaviour. Very few solutions actually fit to that ideally but Raoult's law for the ideal solution is a good enough approximation for the non-ideal solutions that we will continue to use Raoult's law.
Boiling point elevation[edit | edit source]
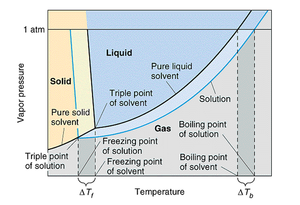
One consequence of Raoult's law is that the boiling point of a solution made of a liquid solvent with a non-volatile solute is greater than the boiling point of the pure solvent (1.2). The boiling point of a liquid is defined as the temperature at which the vapour pressure of that liquid equals the atmospheric pressure. For a solution, the vapour pressure of the solvent is lower at any given temperature. Therefore, a higher temperature is required to boil the solution compare to the pure solvent. This fact is graphically described in a phase diagram for both a pure solvent and a solution of that solvent and a non-volatile solute (Figure 1.1).
In the figure, the vapour pressure of the solution is lower than that of the pure solvent. Because both the pure solvent and the solution need to reach the same pressure to boil, the solution requires a higher temperature to boil. If the difference in boiling point between the pure solvent and a solution is represented as Tb, then the change in boiling point from the following formula can be calculated as:
ΔTb = i·Kb·m (1.2)
In the formula above we use the unit molality for the concentration (m) because molality is temperature independent. The term Kb is a boiling point elevation constant that depends on the particular solvent being used. The term i is the Van't Hoff factor which represents the number of dissociated moles of particles per mole of solute. The Van’t Hoff factor is 1 for all non-electrolyte solutes and equals the total number of ions released for electrolytes. For example, the value of i for Na2SO4 is 3 because that salt releases three moles of ions per mole of the salt.
Freezing point depression[edit | edit source]
As you may have noticed when we looked at the phase diagram (Figure 1.1), the freezing point is depressed due to the vapour pressure lowering phenomenon. In analogy to the boiling point elevation, we can calculate the amount of the freezing point depression with the following formula:
ΔTf = i·Kf·m (1.3)
Note that the sign of the change in freezing point is negative because the freezing point of the solution is less than that of the pure solvent. Just as we did for boiling point elevation, we use molality to measure the concentration of the solute because it is temperature independent.
One way to rationalize the freezing point depression phenomenon without talking about Raoult's law is to consider the freezing process. In order for a liquid to freeze it must achieve a very ordered state that results in the formation of a crystal. If there are impurities in the liquid, i.e. solutes, the liquid is inherently less ordered. Therefore, a solution is more difficult to freeze than the pure solvent so a lower temperature is required to freeze the liquid.
Osmosis[edit | edit source]
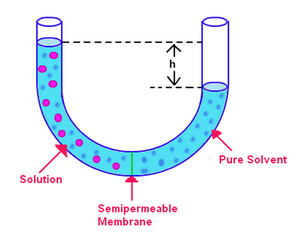
Osmosis refers to the flow of solvent molecules past a semipermeable membrane that stops the flow of solute molecules only (Figure 1.2). When a solution and the pure solvent used in making that solution are placed on either side of a semipermeable membrane, it is found that more solvent molecules flow out of the pure solvent side of the membrane than solvent flows into the pure solvent from the solution side of the membrane. That flow of solvent from the pure solvent side makes the volume of the solution rise. When the height difference between the two sides becomes large enough, the net flow through the membrane ceases due to the extra pressure exerted by the excess height of the solution chamber. Converting that height of solvent into units of pressure gives a measure of the osmotic pressure exerted on the solution by the pure solvent (1.4). p stands for pressure, ρ is the density of the solution, and h is the height of the solution.
p = ρ·h·g (1.4)
You can understand why more molecules flow from the solvent chamber to the solution chamber in analogy to our discussion of Raoult's law (1.1). More solvent molecules are at the membrane interface on the solvent side of the membrane than on the solution side. Therefore, it is more likely that a solvent molecule will pass from the solvent side to the solution side than vice versa. That difference in flow rate causes the solution volume to rise. As the solution rises, by the pressure depth equation, it exerts a larger pressure on the membrane's surface. As that pressure rises, it forces more solvent molecules to flow from the solution side to the solvent side. When the flows from both sides of the membrane are equal, the solution height stops rising and remains at a height reflecting the osmotic pressure of the solution. The equation relating the osmotic pressure of a solution to its concentration has a form quite similar to the ideal gas law:
pV = nRT (1.5)
There p is pressure, V is volume, n is the number of moles, R is the universal gas constant and T is absolute temperature. Although the above equation (1.5) may be simpler to remember, the following form of the equation (1.6) is more useful. This form of the equation has been derived by realizing that n/V gives the concentration of the solute in units of molarity (M, the number of moles of a solute per litre of solution).
p = MRT (1.6)
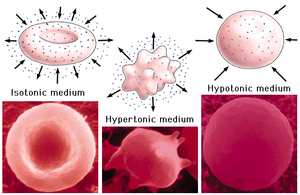
It shows that the osmotic pressure depends on concentration and temperature. There are 3 different sorts of movements caused by osmosis: isotonic, hypertonic, and hypotonic (Figure 1.3).
- Isotonic solution, with equal solute concentration in external and internal liquid, causes no movement.
- Hypertonic solution, with a higher concentration of dissolved substances in external than in internal liquid (within the cell), causes e.g. the cells are shrinking due to osmosis (because the solvent goes out of the cell).
- Hypotonic solution, with a lower concentration of dissolved substances in external than in internal liquid, causes e.g. the cells are swelling and bursting (because the solvent goes into the cell).
COHESION & SURFACE TENSION[edit | edit source]
Cohesion is an intrinsic property of a substance that is caused by the shape and structure of its molecules which makes the distribution of orbiting electrons irregular when molecules get close to one another, creating electrical attraction that can maintain a macroscopic structure such as a water drop. Cohesive attraction (force) is the action or property of like molecules sticking together, being mutually attractive.
The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface. This forms a surface "film" which makes it more difficult to move an object through the surface than to move it when it is completely submersed. The cohesive forces between molecules down into a liquid are shared with all neighbouring atoms. Those on the surface have no neighbouring atoms above and exhibit stronger attractive forces upon their nearest neighbours on the surface. This enhancement of the intermolecular attractive forces (cohesive) at the surface is called surface tension.
Measurement of surface tension focus on cohesive energy present at an interface. The molecules of a liquid attract each other. The interactions of a molecule in the bulk of a liquid are balanced by an equal attractive force in all directions. Molecules on the surface of a liquid experience an imbalance of forces. Surface tension is responsible for the shape of liquid droplets. Although easily deformed, droplets of water tend to be pulled into a spherical shape by the cohesive forces of the surface layer. In the absence of other forces, including gravity, drops of virtually all liquids would be perfectly spherical.
Another way to view surface tension is in terms of energy. A molecule in contact with a neighbour is in a lower state of energy than if it were alone (not in contact with a neighbour). The interior molecules have as many neighbours as they can possibly have, but the boundary molecules are missing (compared to interior molecules) and therefore have a higher energy. For the liquid to minimize its energy state, the number of higher energy boundary molecules must be minimized. The minimized quantity of boundary molecules results in a minimized surface area.