Ionic bond
An ionic bond is a type of chemical bond that occurs between metals and nonmetals with an electronegativity difference greater than 1.7 [1]. During the formation of this bond, the translocation of electrons from one element to another creates oppositely charged ions and their subsequent approach leading to the release of energy and stabilization of the entire system of ion bond formation. An ionic bond containing solids, mostly various salts, e.g. sodium chloride (NaCl) or calcium fluoride (CaF2).
Formation of ions and ionic bonds[edit | edit source]
The elements located in the left part of the periodic table (mainly the elements of the 1st and 2nd groups) are characterised by electropositivity and a low value of ionization energy, which means that they easily split off their valence electrons and form positively charged ions - cations.
These elements include metals. Sodium is an example of such an element:
Na → Na+ + e- (oxidation)
On the contrary, the elements occurring in the right part of the table are typically non-metals. Their common properties include a high value of electronegativity and a large value of electron affinity, due to which they willingly accept electrons and form negatively charged ions - anions.
Chlorine is an example of such an element:
Cl + e- → Cl- (reduction)
As opposite charges are attracted by the electrostatic force, the ions are brought closer together to form an ionic bond.
Na+ + Cl- → NaCl
In the process of formation of ions, one (or more) electrons are detached from the valence layer of the electropositive element and incorporated into the valence layer of the electronegative element. Between the formed ions, the attractive and repulsive forces between cations and anions will subsequently be balanced. This whole process leads to the stabilisation of the particles by reaching the electron configuration of the nearest noble gas, which is the most stable arrangement of electrons of the valence layer.
By shedding an electron, sodium acquires the electron configuration of neon:
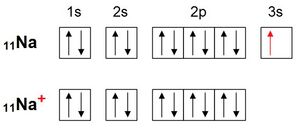
Likewise, chlorine acquires the electron configuration of argon by accepting an electron:
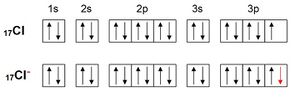
Structure of ionic compounds[edit | edit source]
In contrast to covalent compounds that form single molecules, ionic compounds form typical structures referred to as ionic or crystal lattices. Since ions are spherical bodies, their charge is distributed evenly. As a result, they tend to surround themselves with as many oppositely charged ions as possible. The action of electrostatic forces between oppositely charged ions thus gives rise to the characteristic regular arrangement of cations and anions in space into a three-dimensional crystal structure, the arrangement of which depends mainly on the size of the participating ions. To give an example, in the NaCl crystal, the sodium cation is surrounded by six chloride anions, which in turn are surrounded by six sodium cations.
Crystal lattice of NaCl [4]
Spatial arrangement of ions in NaCl [5]
Spatial arrangement of ions in CaF2 [6]
Properties of ionic compounds[edit | edit source]
At room temperature, ionic compounds are solids with a crystalline structure. They have high melting and boiling points (high dissociation energy) and are poor conductors of electricity. Ionic compounds are well soluble in water and other polar solvents. During dissolution, water molecules interact with an ionic substance, followed by the release of ions from the crystal lattice and surrounding by water molecules (formation of a solvation shell). This process leads to the formation of electrolytes.
By heating solid ionic compounds, by supplying a sufficient amount of energy, bonds between individual ions are broken, resulting in melts capable of conducting an electric current.
Examples of ionic compounds[edit | edit source]
- NaCl – sodium chloride (trivial name: table salt)
- KCl – potassium chloride
- AlCl3 – aluminium chloride
- CaCl2 – calcium dichloride
- CaS - calcium sulphide
- Fe2O3 – iron oxide
- FeO – ferrous oxide
- Hg2Cl2 – mercurous chloride / mercury (I) chloride (trivial name: calomel)
- HgCl2 – mercuric chloride / mercury (II) chloride (trivial names: sulema; corrosive sublimate)
- K2O – potassium oxide
- K2S – potassium sulphide
- Mg3N2
- Br2Mg – magnesium bromide
- MgO – magnesium oxide
- MnO2 – manganese dioxide (pyrolusite)
Links[edit | edit source]
Related articles[edit | edit source]
Bibliography[edit | edit source]
- RUSSELL, John B. General Chemistry. 1. edition. McGraw-Hill, 1992. ISBN 0-07-054445-X.
- SPENCER, James N. (James Nelson) – BODNER, George M. Chemistry : structure and dynamics. 4. edition. Wiley, 2008. ISBN 978-0-470-12928-9.
- CHANG, Raymond – CRUICKSHANK, Brandon. Chemistry. 8. edition. McGraw-Hill, 2005. ISBN 0-07-251264-4.
- JURSÍK, František. Anorganická chemie kovů. 1. edition. Vysoká škola chemicko-technologická, 2002. ISBN 80-7080-504-8.
- FLEMR, Vratislav – DUŠEK, Bohuslav. Chemie I : (obecná a anorganická). 2. edition. SPN - pedagogické nakladatelství, 2007. ISBN 978-80-7235-369-9.
- GÄRTNER, Harald. Kompendium chemie. 1. edition. Euromedia Group - Knižní klub, 2007. ISBN 978-80-242-2012-3.
References[edit | edit source]
- ↑ VACÍK, J., BARTHOVÁ, J., PACÁK, J., STRAUCH, B., SVOBODOVÁ, M., ZEMÁNEK, F. Přehled středoškolské chemie. 4. vydání. Praha: SPN-pedagogické nakladatelství a. s., 1999. 368s. ISBN 80-7235-108-7
- ↑ KOBERA, Jirka. Sodikkationt. Elektronová konfigurace Na+. year uknown. Wikiskripta [online]. <https://www.wikiskripta.eu/w/Iontov%C3%A1_vazba>
- ↑ KOBERA, Jirka. Chloraniont. Elektronová konfigurace Cl-. year uknown. Wikiskripta [online]. <https://www.wikiskripta.eu/w/Iontov%C3%A1_vazba>
- ↑ KOBERA, Jirka. Struktura kuchyňské soli. year uknown. Wikiskripta [online]. <https://www.wikiskripta.eu/w/Iontov%C3%A1_vazba>
- ↑ KOBERA, Jirka. NaCl. year uknown. Wikiskripta [online]. <https://www.wikiskripta.eu/w/Iontov%C3%A1_vazba>
- ↑ KOBERA, Jirka. CaF2. year uknown. Wikiskripta [online]. <https://www.wikiskripta.eu/w/Iontov%C3%A1_vazba>


![Crystal lattice of NaCl [4]](/thumb.php?f=StrukturaNaCl.jpg&width=120)
![Spatial arrangement of ions in NaCl [5]](/thumb.php?f=NaCl_-_sodium_chloride.jpg&width=120)
![Spatial arrangement of ions in CaF2 [6]](/thumb.php?f=Ionvaz1opravdu1.jpg&width=120)