Gamma radiation – mechanism of formation
Gamma radiation is high-energy electromagnetic radiation produced by radioactive and other nuclear and subnuclear events. It is a type of ionizing radiation . It penetrates materials better than alpha radiation or beta radiation , but is less ionizing.
The mechanism of radiation formation[edit | edit source]
Gamma radiation is often produced together with alpha or beta radiation during the radioactive decay of nuclei. When a nucleus emits a α or β particle , the new nucleus may be in an excited state . Gamma radiation can go to a lower energy state by emitting a photon , similar to how an electron in the shell of an atom can go by emitting a quantum of ultraviolet radiation .
Example:
- = electron neutrino
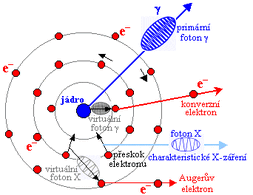
Schematic representation of gamma rays:
Emitters[edit | edit source]
Most radionuclides are mixed emitters - either or . Only some emitters are pure α or pure – radioactive transformation sometimes occurs directly to the ground state of the daughter nucleus (this is the case, for example, with tritium or carbon ). However, pure γ emitters do not exist in nature, but can be prepared using a nuclear reaction in nuclear reactors. (They are used in the field of nuclear medicine ).
Internal radiation conversion[edit | edit source]
Internal conversion of radiation is the process by which the emission of photons radiation is prevented. Their energy is transferred to the electrons of the electron shell and the photon vanishes. The transferred energy is used for the release of the electron and for the kinetic energy with which the electron leaves its place. These electrons are called conversion electrons . An electron from a higher level immediately jumps to its original place, which is accompanied by the emission of X-ray quanta . Even this radiation can undergo internal conversion, the electrons thus emitted are called "Auger electrons " .
Conversion rate[edit | edit source]
= is the relative probability of internal conversion to decay , increases with and with cores.
- Energy of gamma rays
- proton number of an element = number of protons in the nucleus
Isomeric transition[edit | edit source]
An isomeric transition is the transition of a metastable/excited form of a nucleus to a lower energy or ground state. Under certain conditions, the time period of the excited state is long enough that the nucleus exhibits its own radioactivity . Based on this phenomenon, pure emitters can be obtained .
Nuclear isomerism = atoms/nuclei with the same proton and neutron number differing in the energy state of the nucleus.
Links[edit | edit source]
Related Articles[edit | edit source]
External links[edit | edit source]
- Gamma radiation (Czech Wikipedia)
- Gamma radiation (on the Physics in Modern Medicine server))
- Radiobiology (on the server fbmi.sirdik.com)
References[edit | edit source]
- BENEŠ, Jiří – STRÁNSKÝ, Pravoslav – VÍTEK, František. Základy lékařské biofyziky. 2. edition. Karolinum, 2007. 201 pp. ISBN 978-80-246-1386-4.