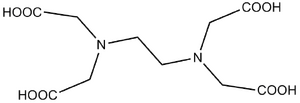
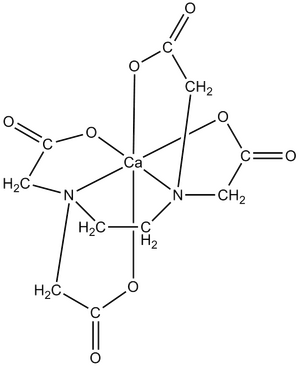
Ethylenediaminetetraacetic acid
From WikiLectures
EDTA and its salts are important chelators . It forms stable water- soluble complexes with transition metals . It is also used, for example, to soften water, as it binds calcium and magnesium and creates soluble compounds with them. In endodontics , it is used to remove the inorganic component of the smear layer during root canal irrigation. Due to its ability to bind calcium cations from the solution, it serves as an anti-coagulant agent in clinical biochemistry. It is also used as a medicine for lead and other metal poisoning .