Electron transport chain
Electron transport chain[edit | edit source]
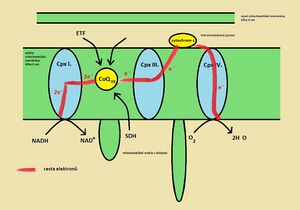
The electron transport chain (the correct Czech term is respiratory chain; the electron transport system is only a literal translation of English texts) is a series of connected redox systems that are located on the inner mitochondrial membrane.' Inner mitochondrial membrane it is very selectively permeable and only small uncharged particles (H2O, O2, CO2 and NH3) pass freely through it. In addition to the enzymes of the respiratory chain, transport proteins for ions and various low-molecular substances (ATP, NADH,...) are also built into this membrane. during the respiratory chain and associated anaerobic phosphorylation (see below)
The respiratory chain processes reduced cofactors supplied from the cytoplasm and the Krebs cycle (mitochondria matrix). These cofactors are reoxidized in the respiratory chain. The set of four enzyme complexes is referred to as Complex I-IV.
Complex I[edit | edit source]
(NADH dehydrogenase, NADH: ubiquinone oxidoreductase) Complex I catalyzes the oxidation of NADH to NAD+ and at the same time ensures the transport of the 2 electrons taken to coenzyme Q (CoQ, ubiquinone). The exact structure of Complex I contains over 40 subunits. Electrons pass through these subunits to ubiquinone, which they reduce to ubiquinol.
Ubiquinol[edit | edit source]
(CoQH2, UQH2) Ubiquinol is the reduced form of ubiquinone. It is an extremely hydrophobic molecule, due to its long isoprenoid side chain, by which it is attached to the inner mitochondrial membrane. In the respiratory chain, it functions as a mobile carrier of electrons from Complex I (II, possibly other enzymes - see below) to Complex III.
Complex III[edit | edit source]
(cytochrome c oxidase, ubiquinol:cytochrome c oxidoreductase) Complex III accepts electrons from reduced CoQ (it is oxidized back to ubiquinone) and transports them (via two cytochromes and an FeS cluster) to cytochrome c.
Cytochrome c[edit | edit source]
Cytochrome c is a small hemoprotein that acts as a mobile electron carrier in the respiratory chain. It is attached to the outer surface of the inner mitochondrial membrane (=located in the intermembrane space but anchored to the inner membrane). Unlike previous enzymes, one cytochrome molecule accepts only one electron. There is a reduction of heme iron from the ferri = III to the ferro = II form. Electrons are therefore transported one by one from Complex III to cytochrome c and then passed on to the last enzyme – Complex IV. Complex III and cytochrome c are collectively called the Q-cycle.
Complex IV[edit | edit source]
(cytochrome c oxidase, cytochrome c:dioxide oxidoreductase) Complex IV takes the electrons from the reduced cytochrome c (it is re-oxidized to the ferric form) and passes them on via 2 cytochromes and 3 copper atoms to the final recipient, which is oxygen. Oxygen accepts 4 electrons and thus two water molecules are formed.
Other inputs to the respiratory chain[edit | edit source]
In addition to NADH, other reduced cofactor molecules also enter the respiratory chain. All these molecules enter the respiratory chain always via CoQ.
Complex II.[edit | edit source]
(succinate dehydrogenase, SDH) - this is an enzyme of the Krebs cycle (the enzyme is composed of FAD - flavin adenine dinucleotide), which catalyzes the oxidation of succinate to fumarate. As it oxidizes succinate, the enzyme molecule itself is reduced = accepts electrons. In order for this enzyme to function again in the Krebs cycle, it must be re-oxidized by transferring the obtained electrons to coenzyme Q. Structurally, Complex II (SDH) is a transmembrane protein, just like Complexes I, III and IV.
ETF[edit | edit source]
ensures the transfer of electrons generated during beta-oxidation to the respiratory chain.
Redox potential and energy transformations[edit | edit source]
Redox potential[edit | edit source]
The redox potential expresses the degree of affinity of substances for electrons'. On the basis of redox potential differences in the respiratory chain, electrons are transported by the above procedure. The redox (more specifically, reduction) potential of NADH is the lowest among the other enzymes of the respiratory chain. Conversely, the affinity of oxygen for electrons is one of the highest. Thanks to this fact, the electrons pass through the enzymes, which gradually increase the value of the redox potential up to oxygen. Therefore, we can label redox potentials as the driving force of the respiratory chain.
Energy[edit | edit source]
Potential energy (potential difference between electrons NADH/FAD/... and oxygen) can be used to do work. As the inner mitochondrial membrane is impermeable to protons under normal conditions, the cell does work when protons are pumped from the matrix into the intermembrane space. Pumping of protons (H+) takes place via Complexes I, III and IV. Overpumping of protons creates a "concentration difference" between the matrix and the intermembrane space (more protons are in the intermembrane space) and thus creates a "gradient". This gradient can later be used by the cell again to carry out work - to synthesize ATP.
Synthesis of ATP[edit | edit source]
ATP synthesis takes place thanks to the enzyme ATP synthase. The latter forms a channel through the inner membrane of the mitochondrion and thus enables the return of protons from the intermembrane space back to the matrix (for the purpose of rebalancing concentrations). Thanks to the flow of protons, part of the ATP synthase spins, which extends into the mitochondrial matrix. The rotation of part of the enzyme is transferred to another subunit (F1), which exerts pressure on the surrounding subunits and thus drives the phosphorylation of ADP to ATP. A specific transporter (ANT – adenine nucleotide translocator) subsequently transfers the newly synthesized ATP molecules in exchange for ADP out of the matrix and into the cytoplasm.
UCP proteins[edit | edit source]
Protons from the intermembrane space can also penetrate the matrix in another way, thanks to UCP proteins (uncoupling protein). However, ATP is not produced in these proteins, and therefore the potential energy of the gradient is converted into thermal energy (we refer to this as inefficient energy transfer). This process is mainly applied in brown adipose tissue (found in newborns in the area of the shoulder blades and kidneys), where we find the UCP 1 protein (uncoupling protein 1, thermogenin) in the inner mitochondrial membrane.
Links[edit | edit source]
References[edit | edit source]
- LEDVINA, Miroslav. Biochemie pro studující medicíny. 2. edition. Praha : Karolinum, 2009. 548 pp. pp. 85-90. ISBN 978-80-246-1414-.
- učební text Dýchací řetězec a tvorba ATP 3. lékařské fakulty UK, dostupné z http://fblt.cz/skripta/ii-premena-latek-a-energie-v-bunce/7-dychaci-retezec-a-tvorba-atp/
- přednáška Dýchací řetězec a tvorba ATP, dostupný z https://www.youtube.com/watch?v=OtoPAghbymQ