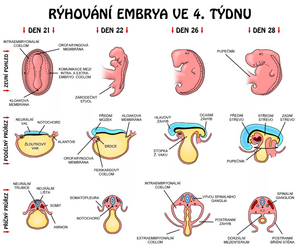
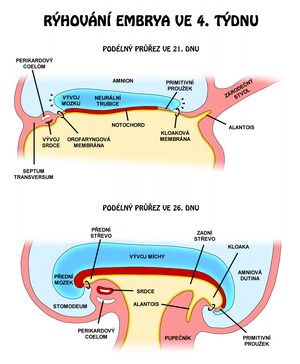
Development of the spinal cord
The basis of the central nervous system is at the beginning 3. weeks plate of thickened ectoderm, neural plate. It has the shape of a slipper lying in the midline on the dorsal side of the embryo in front of the primitive (Hensen's) node. The lateral edges of the disc rise to form neural crests. The neural crests move closer together until they eventually fuse to form the neural tube. Fusion begins in the cervical region and continues in both the cranial and caudal directions. Suddenly, the neural crests begin to fuse together, the neural tube has openings called ``neuroporus anterior et posterior. Neuropore closure proceeds cranially and caudally, and at the 18–|20 somite stage complete closure of the anterior neuropore occurs (day 25), closure of the posterior neuropore takes place two days later.
- At the cephalic end of the neural tube, 3 primary brain vesicles are formed
- forebrain or prosencephalon;
- middle brain or mesencephalon;
- hindbrain or rhombencephalon.
- 2 bends are formed at the same time
- occipital flexure or flexura cervicalis - boundary of the hindbrain and spinal cord;
- parietal flexure or flexura cephalica - area of the midbrain.
- Changes in week 5
- Prosencephalon consists of 2 parts:
- telencephalon or terminal brain;
- diencephalon or midbrain.
- Mesencephalon.
- Rhombencephalon:
- metencepahalon;
- myelencephalon.
The spinal cord (medulla spinalis) has a canal (canalis centralis) inside, which continues into the cavity in the brain sacs:
- in rhomboencephalon – 4. ventricle;
- in the diencephalon – 3. ventricle;
- in the telencephalon, respectively cerebral hemispheres - lateral cerebral ventricles are connected to the third ventricle by the foramen interventriculare (Monroi);
- in mesencephalon – aquaeductus mesencephali (Sylvii).
Layers of the Spinal Cord[edit | edit source]
- Neuroepithelial layer
- Neuroepithelial cells form the wall of the newly formed neural tube, canalis centralis. These cells penetrate the entire wall of the neural tube, and even before and especially after the closure of the neural tube, they divide rapidly and their number increases massively. This creates a thick layer of multi-rowed neuroepithelium.
- After the tube is closed, the next generation of cells differentiates from the neuroepithelial cells, characterized as cells with a large light nucleus and a dark nucleus - precursor cells of neurons, neuroblasts.
- Mantle layer
- It is produced by neuroblasts.
- It is around the periventricular neuroepithelial layer.
- Later, gray matter is formed from it.
- Marginal layer
- Contains nerve fibers emerging from neuroblasts of the mantle layer.
- It acquires a whitish appearance due to myelination - the white matter of the spinal cord.
Spinal discs[edit | edit source]
Due to the constant multiplication of neuroblasts in the mantle layer of the neural tube, it thickens at the ventral and dorsal edges.
Basal plate - ventral thickening
- contains the differentiating motoneurons of the anterior horns of the spinal cord - the motor area of the spinal cord.
Alar plate - dorsal thickening
- sensitive area of the spinal cord.
The boundary between the alar and basal plates is the sulcus limitans.
The parts of the neural tube lying in the midline dorsally are referred to as the ceiling plate and the parts lying ventrally as the basal plate are devoid of neuroblasts; contain crossing nerve fibers.
Between the areas of the basal and alar plates, a group of neurons differentiates, which form a smaller lateral horn - this is a group of neurons, projecting into the sympathetic part of the autonomic nervous system and which are formed only in the thoracic (Th1-Th12) and upper lumbar (L1-L3) parts spinal cord.
Histogenesis of the neural tube[edit | edit source]
Neurons[edit | edit source]
- they come from neuroblasts, which arise from the division of neuroepithelial cells. First, they have a central process that faces the lumen of the central canal as a transitional dendrite, travels into the mantle layer along the radially arranged glial cells, this fiber disappears, and the neuroblast is temporarily round and without a process - an ``apolar neuroblast. Later, it differentiates into two new cytoplasmic protrusions on opposite sides of the cell body – bipolar neuroblast. One of the projections grows faster to form a primitive axon and the projection on the opposite side branches to form primitive dendrites - a multipolar neuroblast' that develops into a mature neuron. It loses the ability to divide.
Axons of basal plate neurons penetrate the marginal zone and emerge on the ventrolateral periphery of the spinal cord. They conduct motor impulses from the spinal cord to the muscles and together form the 'front root of the spinal nerves.
''Axons of neurons of the alar plate' penetrate the peripheral zone of the spinal cord and there run either ascending or descending to different levels of the spinal cord and become association and projection neurons and interneurons.
Glial cells[edit | edit source]
- They come from glioblasts' which arisethey originate from neuroepithelial cells. They migrate from the neuroepithelial periventricular layer to the mantle layer, where they differentiate into two types of astrocytes – plasma and fibrillar astrocytes. Oligodendrocytes also originate from glioblasts, migrate to the marginal zone and form the myelin sheaths of descending and ascending axons passing through this marginal zone.
- Microglia - they have a great ability to phagocytose, they arise from mesenchyme cells that were transported to the CNS together with growing blood vessels.
- In the periventricular zone, there are cells that have not traveled and differentiate into ependymal cells, lining the cavities of the CNS. In the perivetricular zone there are also neural stem cells'.
Neural crest cells[edit | edit source]
- During neurulation, they differentiate from neuroectoderm along neural crests. They arise along the entire length of the neural tube, except for the rostral parts of the prosencephalon. They arise from:
- spinal ganglia on the posterior roots of the spinal nerves;
- postganglionic neurons of the autonomic nerves;
- adrenal medulla cells;
- Schwann cells;
- melanocytes;
- odontoblasts;
- part of the brain casings;
- ectomesenchyme of the gill arches.
- In further development, the neurons of the spinal ganglia form two projections:
- Centrally directed processes (centripetal) enters the spinal cord on its dorsolateral circumference, forms the posterior root of the spinal cord and ends either in the posterior spinal horn or rises through the marginal zone into the medulla oblongata.
- Peripherally growing projections (centrifugal) attach to the ventral roots of the spinal nerves and together form the spinal nerve. These projections end in the periphery in sensitive receptors.
Spinal nerves[edit | edit source]
In the 4th week, motoneuron axons grow from the anterior horns of the spinal cord and form 'anterior spinal roots.
The posterior spinal roots are formed by a collection of fibers arising from spinal ganglion cells. The centrifugal processes join the ventral roots of the spinal nerves and together form the spinal nerve. Shortly thereafter, it divides into an anterior and posterior branch.
Myelination[edit | edit source]
The myelin sheath of peripheral nerves is formed by Schwann cells. These originate from the neural crest and migrate to the periphery. They wrap around the axons (myelin lamellae are formed) and from the beginning of the 4th prenatal month form Schwann's myelin sheath.
The myelin sheath of nerve fibers in the CNS is formed by oligodendrocytes, which, unlike Schwann cells, participate in the myelination of more axons.
Some axons coming from the higher divisions of the brain to the spinal cord are not yet myelinated at the end of the 1st postnatal year. CNS pathways begin to myelinated only at the time when they begin to be functionally applied.
Changes in the position of the spinal cord in the spinal canal[edit | edit source]
In the 3rd month, the spinal cord lies along the entire length of the spinal canal and the nerves exit into the intervertebral foramina at the level of their exits from the spinal cord. During development, the spinal canal together with the dura mater elongates much faster than the spinal cord, so its end gradually reaches a higher and higher level.
- In the newborn in the L3 area,
- in adulthood, level L2 – L3and a thin fiber - filum terminale - continues. It is covered only by the pia mater and attaches to the periosteum of the coccyx. The saccus durae matris and the subarachnoid space continue up to the S2 level.
The result of this growth is the oblique exit of the root fibers of the spinal nerves from the intervertebral foramen in a caudal direction. Under the end of the spinal cord it forms a root bundle - 'cauda equina.
The dura mater remains fixed to the walls of the spinal canal and does not move with the spinal cord.
Links[edit | edit source]
Related Articles[edit | edit source]
Literature[edit | edit source]
- SADLER, Thomas W. Langmanova lékařská embryologie : Překlad 10. vydání. 1. edition. Grada Publishing, a. s, 2011. 432 pp. ISBN 978-80-247-2640-3.
- MOORE, Keith L – PERSAUD, T.V.N. Zrození člověka : Embryologie s klinickým zaměřením. 1. edition. 2000. 564 pp. ISBN 80-85866-94-3.
- SADLER, T.W. Langman's Medical Embryology. 10. edition. 2006. 385 pp. ISBN 978-0-7817-9485-5.