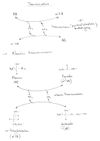
Common mechanisms of amino acids conversion, deamination, transamination, nitrogen balance
Transamination[edit | edit source]
It is a process present in the amino acid metabolism where the amino group is transferred from the amino acid to a certain α-ketoacid with the consequent formation of a second α-ketoacid and amino acid. The reaction is catalyzed by the enzyme aminotranferase (aka transaminase) which requires pyridoxal phosphate as a prosthetic group. All transaminases contain this prosthetic group which derives from pyridoxine a water soluble vitamin also known as vitamin B6. The amino group from amino acids is temporarily uptaken by the pyridoxal phosphate as pyridoxamine phosphate prior to its donation to an α-ketoacid. All aminoacids except lysine, threonine, proline and hydroxyproline participate in transamination process.
Deamination[edit | edit source]
It is a process occurring in the liver during the metabolism of amino acids. The amino group is removed from the amino acid and converted to ammonia-NH3 whose toxic activity is canceled by conversion into urea which is eventually excreted. The glutamate dehydrogenase-GDH enzyme occupies a central role in nitrogen metabolism. Glutamate amino acid is cleaved into α-ketoglutarate and ammonia a reaction catalyzed by GDH in a process called deamination. Glutamate is the only amino acid that undergoes oxidative deamination at a relatively high rate. Thus, the formation of ammonia mainly occurs via glutamate deamination.
Nitrogen balance[edit | edit source]
- urea is the end product of the ammonia detoxification pathway. Toxic ammonia is a side product of protein metabolism and bowel bacterial metabolism.
- the concetration of urea in blood correlates with the state of protein metabolic functions of the liver, function of the kidney and the level of the total body hydration.
- the protein turnover depends on the nutritional condition of the body. It is closely related to the metabolism of saccharides and fatty acids. The intensity of the proteocatabolic processes and the intensity of proteoanabolic processes is similar, only if the supply of proteins and energy sources in the form of saccharides and free fatty acids is sufficient.
- on the contrary, when the supply of saccharides, free fatty acids and essential amino acids is short, the proteocatabolic processes overweight. In this situation the proteins are exclusively degraded in order to provide the amino acids necessary for gluconeogenesis. In gluconeogenesis only the carbonic skeletons of amino acids are required and thusly the amino group ought to be removed.
- the removal of the amino nitrogen occurs during the process of transamination and oxidative deamination. Increased deamination results in an increased production of ammonia which is consumed in the urea cycle. Urea, a hydrophilic non-toxic compound, is excreted by the kidneys into the urine. Consequently, the amount of urea excreted in urine correlates with the rate of protein metabolism. Thus the nitrogen balance is an indicator of nutritional state of an individual.
- Considering adult, healthy individual the normal excretion of urea in urine ranges from 400 to 500 mmol/day.
Links[edit | edit source]
Bibliography[edit | edit source]
MURRAY, Robert K. – BENDER, David A.. Harper's Illustrated Biochemistry. 29th edition. McGraw-Hill Companies, Inc., 2012. ISBN 978-0-07-176576-3.